Chemistry Reference
In-Depth Information
maxima of excitation λmax
ex
= 360 nm and emission λmax
em
= 435 nm. According to the
data of the work [24] fluorescence, characterized by λmax
ex
=365 nm and λmax
em
=445 nm
should be classified as fluorescence of impurities - products of PA oxidation and initial
monomers.
These data agree quite well with conclusions of the work [22] in which it was noted that
change of fluorescence intensity happens simbatly with the change of quaintity of formed
C=0 bonds. Similar results were got by Allen and Makk-Kellar [23] while studying
fluorescence of model compounds and nylon - 6,6. The authors explain the nature of polymer
luminescence by the presence of fragments, being in the main polymer chain, consisting of
C=0 groups with conjugated double bond of ethylene type.
Formation of these groups is observed mainly at the stage of polycondensation and also
fiber formation [24]. Their identification was carried out according to ultra- violet spectra and
spectra of fluorescence, moreover main groups were oligoenimic with the content up to 44
mole/kg.
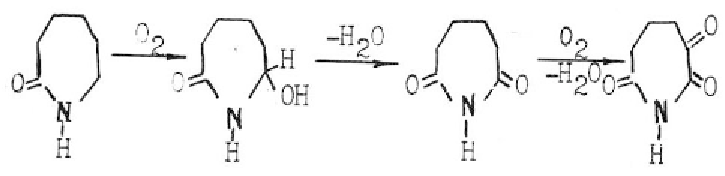
Interesting results are got in the work [25] in which the authorse suppose that
luminescence of PCA may be attributed to the presence of compounds of keto-imide structure
in the polymer, these compounds being characterized by λmax
ex
=356 nm and λmax
em
417
nm, connecting the formation of these products at the stage of polymerization of ε-
caprolactam according to the following scheme:
This point of view is shared by Postnikov and his research workers [12, 26], who
consider that keto-imide compounds may be formed even at the early stage of PA production.
They were the first to construct the simplest scheme of reaction capable to describe
mechanism of PA photooxidation taking into account formation and consumption of keto-
imide compound:
where X- keto-imide; A- intermediate product, preceding keto-imide, r*- light radical of
HO
2
*, O*H type.
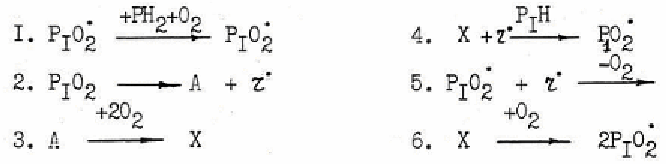

Search WWH ::

Custom Search