Chemistry Reference
In-Depth Information
Literature survey has shown, that there are no data on the effect structure of
introducticing modifiers on physico-chemical properties of polymer and also in what classes
of chemical substances it is necessary to search for potential light-thermal stabilizers.
As it is shown earlier, effective additives-modifiers of polyfunctional action may be
compounds, containing conjugated systems (CS) with high degree of conjugation.
Hexaazocyclanes (HC), having developed conjugation chain, high photo and thermal
stability, have been chosen as the objects of investigation. Presence of chromophore groups
allows to use them in addition as polymer dyes.
Compounds, presented in Figure 3.1, were synthesized on the basis of phthalodimitrile
(1); π-phenyldiamine (2) and derivatives of diaminefluorene (3). In order to check the effect
of macrocyclic stabilizer structure on HC properties there were studied substances being the
products of condensation of phthalodinitrile (1) with m-phenyldiamine (4).
Chemical structures of chosen hexaazocyclanes differ in degree of conjugation.
NH
2
NH
2
C
=
C
C
=
NH
2
NH
2
NH
2
NH
2
(1)
(2)
(4)
(3)
Characteristics of obtained compounds are given in Table 27.
Table 27. Characteristics of hexaazocyclanes
Conventional
sign
Medium
molecular mass
λ
max
, nm
(solvent acetone)
Melting
T,
0
C
Decay
T,
0
C
Colour of the
dye
HC-1
600
360
305
330
yellow
HC-2
580
380
327
345
lemon
HC-3
670
340
315
327
yellow
HC-4
454
400
345
330
beige
HC-5
554
330
335
365
yellow
HC-6
552
360
327
350
beige
HC-7
538
330
300
347
light-brown
Electronic absorption spectra of compounds being used contain absorption band with
λ
max
=330-400 nm (Figure 3.2).
Criteria of compounds thermal stability are temperature values: melting, beginning of
decay and losses of system initial mass at differential-thermal analysis (DTA) by 5, 10, 25%.
These data are given in Table 28.
As it is seen from Table 28 all the compounds under investigation possess high thermal
stability, exceeding greatly thermal stability of PETP. Temperatures of melting and beginning























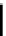

























Search WWH ::

Custom Search