Biology Reference
In-Depth Information
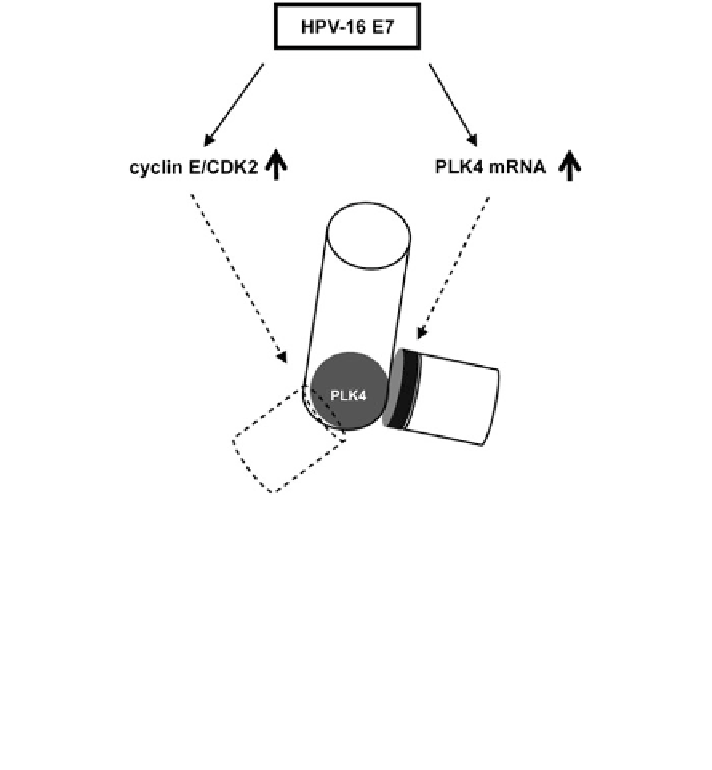
Fig. 12.2 HPV-16 E7 and the complexity of centriole duplication control. HPV-16 E7 induces
the formation of an S-phase-like milieu through binding and degradation of pRB-family
members, interaction with histone deacetylases (HDACs), and inactivation of the CDK inhibitors
p21
Cip1
and p27
Kip1
, ultimately promoting the deregulation of E2F-mediated gene transcription
and the aberrant activation of cyclin E/CDK2 complexes. Cyclin E/CDK2 can promote centriole
multiplication through the aberrant recruitment of PLK4 protein to maternal centrioles; however,
it is currently not clear whether this is through a direct effect on PLK4 or through another protein.
The increase of PLK4 mRNA expression in HPV-16 E7 expressing cells is likley to be crucial for
the induction of centriole multiplication. PLK4 protein level is normally restrained at maternal
centrioles by cellular proteolytic mechanisms. Therefore, HPV-16 E7 expression may also
interfere with the proteolytic control of PLK4 protein level to induce aberrant daughter centriole
formation, a hypothesis that warrants further testing
is highly unstable and it is also possible that HPV-16 E7 may interfere with post-
translational regulatory mechanisms to further increase PLK4 abundance and/or
stability at maternal centrioles.
12.13 Additional Mechanisms of HPV-16 E7-Induced
Centrosome Abnormalities
As mentioned earlier, HPV-16 E7 can also induce centrosome overduplication in a
pRB-independent manner. The first evidence of this came from the observation
that, in contrast to full-length wild-type HPV-16 E7, the HPV-16 E7 mutant
construct, D21-24, was unable to induce centriole overduplication in both normal