Environmental Engineering Reference
In-Depth Information
N
V
25 μm
V
C
N
P
D
25 μm
50 μm
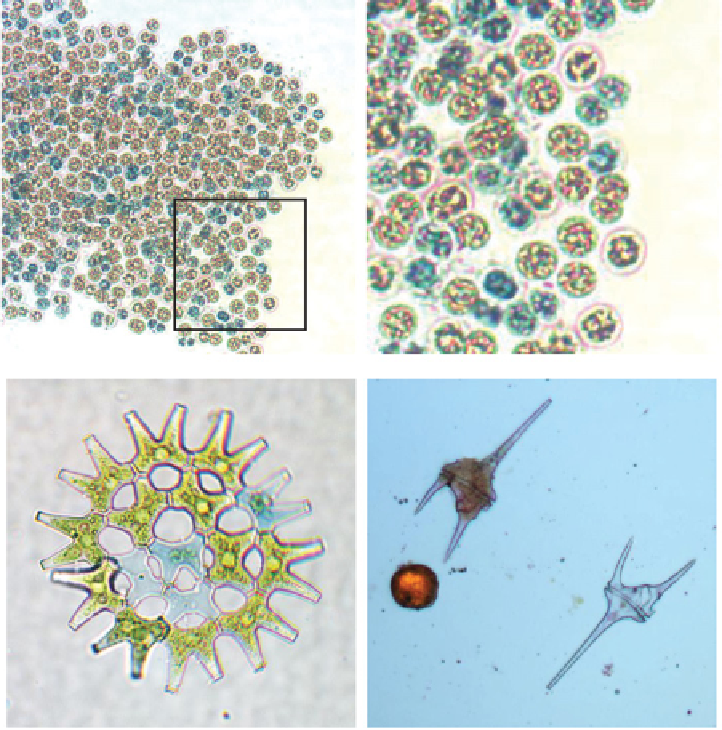
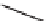
Figure 2.18
Observation of viable and non-viable algal cells in Evans Blue-stained preparations of freshly isolated
phytoplankton. Top: Low-power and detailed views of
Microcystis
colony showing a mosaic of stained (non-viable:
N) and unstained (viable: V) cells. Bottom left: Colony of
Pediastrum
with distinct non-viable cells, some with the
remains of cell contents. Bottom right: Live cells (with dense contents) of
Ceratium
(C) and
Peridinium
(P), plus a dead
(completely empty) cell of
Ceratium
(D).
(Fig. 2.18). In other cases, the dead cells may have
totally disintegrated, leaving a decomposing mass of
organic debris (Fig. 2.7). Another direct indication
of dead cells within the lake is obtained by
in situ
fluorimeter traces of chlorophyll-
a
and particulate
matter (Fig. 2.10), where there is a clear lag in the
particulate trace compared with chlorophyll. In this
situation, dead cells have lost their internal biomass
(including chlorophyll) but remain in suspension as
they sediment out of the surface waters of the water
column.
The phase of greatest phytoplankton population
decline and cell death in temperate eutrophic lakes
occurs towards the end of the summer bloom, when
non-viable cells, PCD and dead cells are particularly
evident. At this time the lake is still stratified, and
senescent changes are triggered primarily by adverse
environmental alterations within the epilimnion.
In addition to its ecological importance, the occur-
rence of senescence and cell death also has practical
implications for the determination of biomass. The
partial or complete loss of cell contents means that














Search WWH ::

Custom Search