Environmental Engineering Reference
In-Depth Information
(actively photosynthetic, vegetative cells) to benthic
form (resistant spores) begins in the upper part of the
water column, and algal spores can be collected in
sediment traps (Fig. 2.7) as they begin their descent
to the lower part of the water column. These overwin-
teringbenthicpopulationsarepresentbothonshallow
and deep (
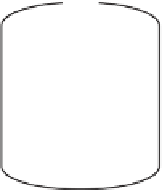
Detachable bottle
collects algae moving
upward
Plastic container
(open at bottom)
WATER
COLUMN
10 m) sediments, the latter well below the
photic zone, and can be sampled by direct collection
of bulk sediment samples or via recruitment traps.
>
Mesh-covered
side vents allow
circulation
Bulk samples
SEDIMENT
Attachment
spike
Bulk samples of sediment algae can be obtained using
either a grab sampler (removing a parcel of sediment
- e.g. Ekman or Peterson grabs) or a core sampler
(removing a cylinder of sediment). Sediments are
often very fine, so samples need to be taken as care-
fully as possible to make sure that the substratum is
not disturbed and that organisms are retained
in situ
.
In their studies on
Microcystis
, Verspagen
et al
.
(2005) collected core samples of lake sediment,
using a Perspex corer. The depth of sample was
adjusted to collect a core of sediment that did not
extend beyond the surface algal layer - ranging
from 2 cm (shallow parts of the lake) to 8 cm
(deep regions). Subsequent laboratory analysis of
the sediment involved homogenisation, suspension
in mineral liquid medium followed by centrifuga-
tion - leaving cells and colonies of
Microcystis
in
the supernatant. A purified suspension of
Microcystis
was then obtained via flow cytometry, with cytomet-
ric selection based on particle size (0.5-2000 μm)
and fluorescence (phycocyanin). The biomass of lake
sediment populations were compared with those from
surface waters and sedimentation traps in relation to
dry weight and chlorophyll-
a
.
Microscopic examination of sediments, using sim-
ilar procedures to those with phytoplankton, is also
important for counting benthic populations. Popu-
lations of sediment surface akinetes of
Anabaena
(Karlsson-Elfgren and Brunberg, 2004; Baker, 1999)
and
Gloeotrichia
(Karlsson, 2003) have been esti-
mated in this way. In a related laboratory study,
akinetes of
Gloeotrichia
were isolated from sedi-
ments by Karlsson (2003) using a Pasteur pipette to
determine the sediment growth period.
Figure 2.22
Phytoplankton recruitment trap - used to
collect benthic algae that are rising from the lake sedi-
ment.FigureredrawnandadaptedfromKarlsson-Elfgren
and Brunberg, 2004.
Recruitment traps
The vertical release of benthic algae into the water
column(recruitment)hastypicallybeenstudiedusing
samplers modified from sedimentation traps (turned
upside down). The traps used by Karlsson-Elfgren
and Brunberg (2004), for example, were large 20 l
vessels - open at the bottom and anchored to the sedi-
ment by long spikes (Fig. 2.22). Deep traps were also
attached to an anchor, to which a surface buoy was
connected. To allow lateral exchange of water, but not
the studied algal species, two openings were cut into
the side of the vessel and covered by 40-μm mesh. On
top of each trap, a 500 ml plastic bottle filled with fil-
tered (40 μm mesh) lake water was attached to collect
algal filaments moving upward. Sample bottles were
collected by divers on a weekly basis, and replaced
with new ones containing filtered lake water.
Thesuspensionofalgaecollectedfromrecruitment
traps can be analysed in terms of biomass and species
composition as with phytoplankton samples.
2.7.2 Benthic algae and sediment stability
Analysis of benthic algae is an important aspect of
investigations on sediment erosion. The studies of










































Search WWH ::

Custom Search