Biology Reference
In-Depth Information
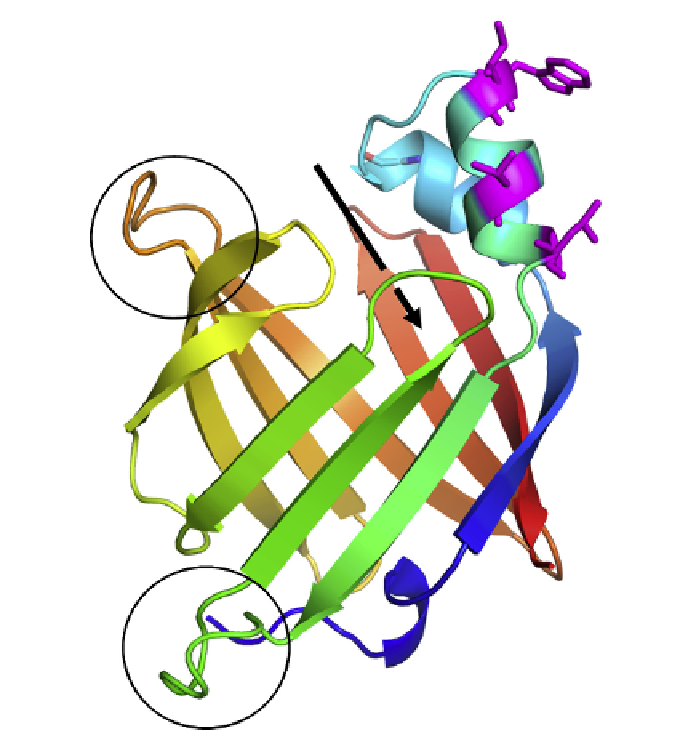
FIGURE 3.9
Structure of the As-p18 protein of the perivitelline fluid of
Ascaris
eggs.
The crystal structure of As-p18, which is essentially identical to the NMR structure of the
protein, showing the extra loop region (lower circle) at the opposite end of the molecule
from the presumed portal of entry for lipid ligands.
108
The position of the presumed portal
of entry of ligands into the binding cavity underneath the two short helices is indicated by
the arrow. The slightly enlarged loop adjacent to the portal is indicated by the upper circle.
The unusually exposed apolar side chains projecting into solvent near the portal region are
shown in magenta and with the side chains added (tryptophan, methionine, isoleucine,
valine, and a leucine); clusters of this kind are typical of those cytosolic fatty acid binding
proteins that interact collisionally with membranes in the process of exchanging lipid
ligands.
99,100,103
Structural coordinates made available by Dr Mads Gabrielsen. For full color
version of this figure go to
www.gla.ac.uk/nematodes
surrounding solvent water. The only FABP that does not perform this way
(liver FABP) instead has a cluster of charged amino acids in this relative
position.
103
This could mean that As-p18 also interacts with membranes
by direct contact in order to collect or deliver lipids, although it remains