Biology Reference
In-Depth Information
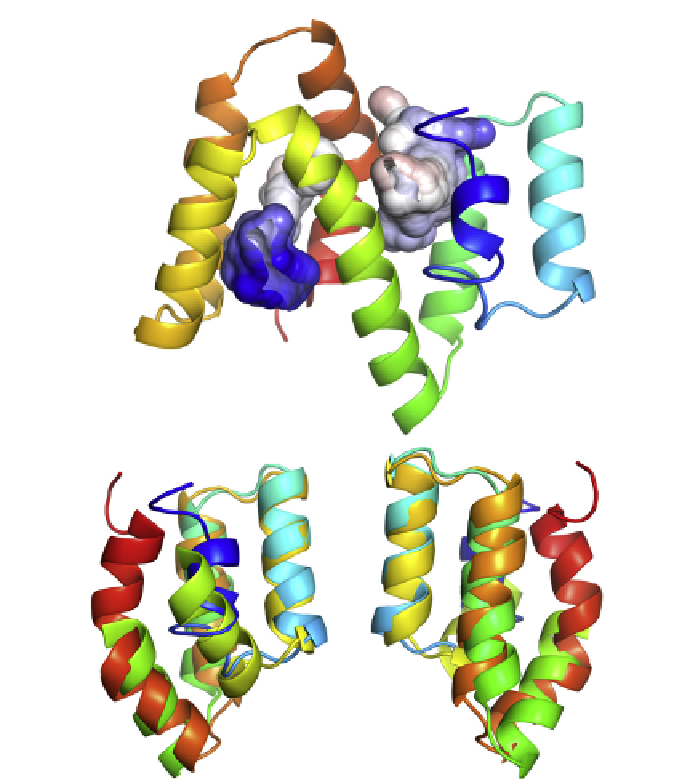
FIGURE 3.8
The molecular structure of ABA-1. A ribbon representation of ABA-1A as
solved by protein nuclear magnetic resonance. The protein is helix-rich, and has two
binding cavities, the internal surfaces of which are predominantly apolar as befits
a hydrophobic binding pocket. The surfaces of the cavities are shown colored according to
electrostatic potential (blue, positive, graded through white, apolar, to red, negative). The
protein structure comprises two domains joined by the long central helix, and the structures
of these two halves of the molecule are virtually superimposable, supporting the idea that
each unit of these polyproteins is itself derived from an ancient duplication event. The lower
two panels show a superimposition of the two halves of the protein from different view-
points, illustrating the similarities in the structures of the two domains. For a full color version
of this figure go to
www.gla.ac.uk/nematodes