Biology Reference
In-Depth Information
FIGURE 4
Comparison of non-
ergodic and threshold dissociation
techniques. (A) Illustration of frag-
mentation sites for threshold and
nonergodic techniques. (B) Fragment
ion map of bovine ubiquitin demon-
strating a greater number of
b
(A)
c
H
R
H
C
N
N-terminus
C-terminus
C
inter-
O
H R
residue cleavages for ECD (red
ags)
Z
.
than for CAD (blue
ags).
(B)
y
be achieved in automated top-down work
ows
that accounted for target protein intensity, mass,
and charge in selection of MS/MS conditions.
39
Nonergodic dissociation techniques employ
exothermic processes that initiate when low-
energy electrons (~5 eV) supplied by a cathode
(electron-capture dissociation [ECD])
63
or chemi-
cally ionized reagents (electron-transfer dissocia-
tion [ETD])
68
are captured by the protein, forming
a radical cation that undergoes rapid rearrange-
ment and cleaves the backbone before the energy
can be dispersed throughout the protein. Frag-
mentation in ECD and ETD occurs along the
protein backbone amine (N-C
biomolecule PTMs such as glycosylation, phos-
phorylation, oxidation, and protein acylation.
69
As with threshold techniques, precursor charge
state directly affects protein dissociation;
higher-charge ions tend to dissociate with higher
ef
ciency.
70,71
ECD/ETD are amenable to high-
throughput characterization of peptides with
online LC/MS acquisition events;
59,72
however,
for
large proteins the methods are largely
con
ine infusion experiments because
the low product ion signal-to-noise ratios (S/N)
necessitate extensive spectral averaging.
ned to of
) bonds, forming
N- and C-terminal product ions (denoted
c and z
.
ions, respectively; see
Figure 4
A).
65
Non-
ergodic approaches with proteins typically
produce few alternative fragment ions (e.g., side
chain losses and the formation of a
.
/y fragments)
and neutral losses, and PTM losses are seldom
observed. Additionally, electron capture occurs
randomly along the protein backbone, resulting
in minimal preferential fragmentation channels
compared to threshold techniques (
Figure 4
B).
The random nature of ECD and ETD provides
improved sequencing information, making the
techniques
a
SAMPLE PREPARATION AND
SEPARATIONS
MS analysis of intact proteins presents unique
challenges owing to factors that lead to signal
suppression during ESI, including factors such
as the numbers of proteins in the sample, physi-
cochemical properties of proteins (e.g., mass
range, pI, and hydrophobicity), heterogeneity
of protein conformations, modi
cation states,
protein solubility, and sources of chemical
noise.
73
Additionally, protein heterogeneity and
charge multiplicity can further
ideally suited to characterizing
reduce the

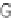



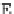

































































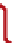
























































Search WWH ::

Custom Search