Environmental Engineering Reference
In-Depth Information
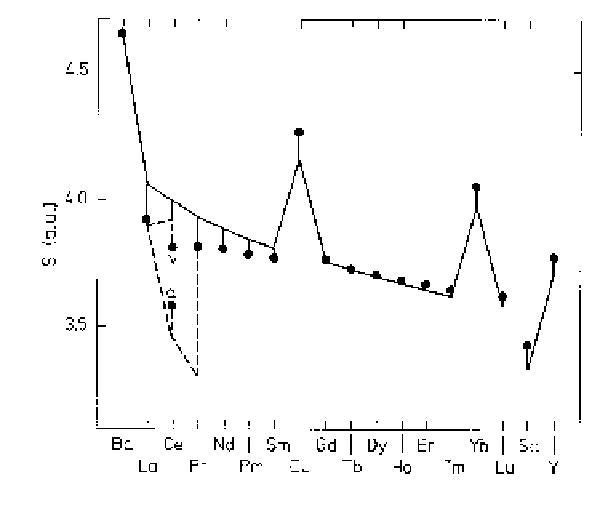
Fig. 1.14.
The equilibrium atomic radii for the rare earth metals, after
Skriver (1983). The full circles indicate the experimental values. The full
line is a calculation including the
s
,
p
,and
d
partial pressures, while the
broken line indicates that the
f
contribution is also taken into account.
screened increase in the nuclear charge, which leads to the lanthanide
contraction. This contraction is clearly apparent in the atomic radii
shown in Fig. 1.14. The values calculated from the condition that the
total pressure is zero agree very well with the experimental observations
for the heavy metals, but if the
f
contribution is neglected, the calcu-
lated electronic pressure is increasingly too high as the atomic number
decreases. As mentioned earlier, the partial pressure of the
f
band is es-
sential for understanding
α
-Ce, and it seems that the interaction of the
f
electrons with their surroundings makes a contribution to the binding,
even in some metals in which the magnetic behaviour strongly indicates
that they are localized.
In Eu and Yb, the intra-atomic interactions make it favourable to
(half) fill the sub-band by transferring an electron from the conduction
bands to an
f
state, leading to the formation of the divalent cubic struc-
tures which strongly resemble the alkaline earth metals. This transfer
occurs predominantly at the expense of the
d
electrons, whose bind-
ing contribution to the electronic pressure is thereby reduced, causing
Search WWH ::

Custom Search