Environmental Engineering Reference
In-Depth Information
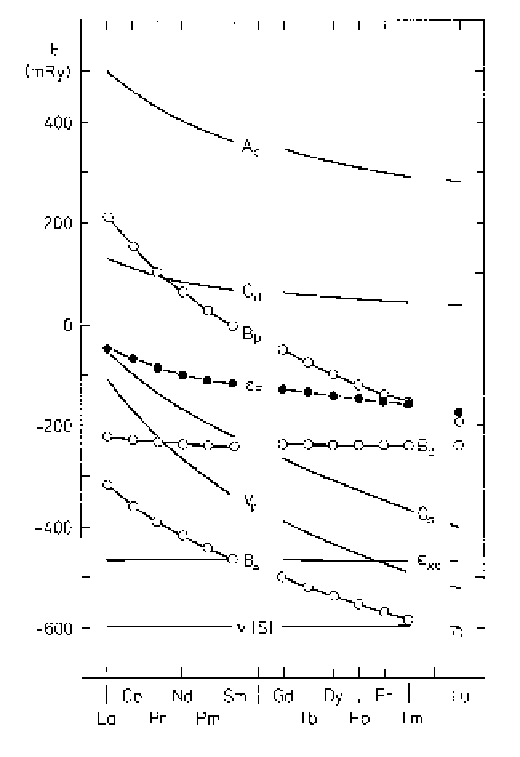
Fig. 1.12.
Characteristic band energies for the trivalent lanthanides,
for a common value of the atomic radius
S
, after Skriver (1983). The
values of the potential
v(S)
and the exchange-correlation energy
ε
xc
at
the atomic sphere are shown, together with the bottom,
B
l
,thecentre,
C
l
,andthetop,
A
l
,ofthe6
s
,6
p
,and5
d
bands, and the Fermi level
ε
F
. The relative lowering of the 6
s
band with increasing atomic number
reduces the 5
d
occupancy, which in turn changes the crystal structure.
see, for the crystal structure. The reason for the fall in the band en-
ergies is the increase of the nuclear charge with atomic number, which
is incompletely screened by the additional
f
electrons. The potential
v
eff
(
r
) in (1.2.12) is therefore on average increasingly negative, and in
order to maintain an unchanged boundary condition, as expressed by
the logarithmic derivative, the band energies must decrease accordingly.
Search WWH ::

Custom Search