Environmental Engineering Reference
In-Depth Information
field, rather than the Schrodinger equation, but it may be more instruc-
tive to consider them as perturbations which, to order (
p/mc
)
2
,augment
the one-electron potential with
p
4
8
m
3
c
2
−
h
2
4
m
2
c
2
dv
dr
∂
∂r
+
1
2
m
2
c
2
r
dv
dr
s
·
l
.
−
(1
.
2
.
13)
The first term, which is due to the increase of mass with velocity, reduces
the energy of all states by an amount which decreases with
l
, while the
second 'Darwin' term increases the energy of
s
states only. These effects
may both be incorporated into the central field, but the last term couples
together the spin and orbital motion in a way that has far-reaching
consequences for the magnetic properties.
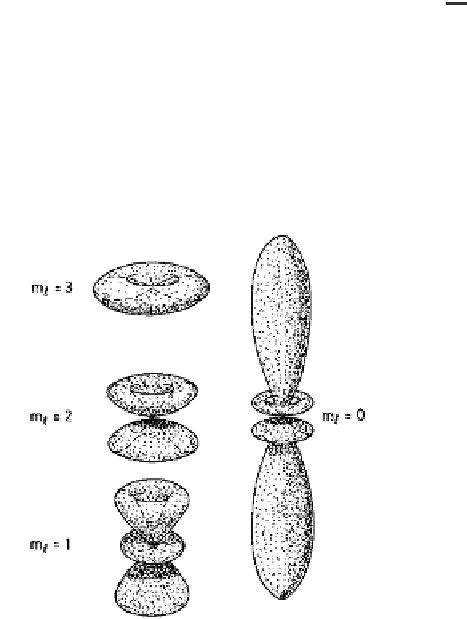
Fig. 1.2.
The angular variation of
the 4
f
wavefunctions. The interac-
tion of the highly anisotropic charge
clouds with the crystalline electric
fields gives rise to the large single-
ion anisotropies observed in the rare
earth metals.
In the Russell-Saunders coupling scheme, which is an accurate pro-
cedure for the 4
f
electrons, the spins
s
i
of the individual 4
f
electrons
are coupled by the exchange interaction, diagonal in the total spin
S
of the incompletely filled subshell, while the Coulomb interaction simi-
larly combines the
l
i
into the total orbital momentum
L
.Intermsofthe
one-electron functions, the wavefunction for the subshell may be written
Ψ(
LSM
L
M
S
)=
m
l
m
s
C
(
LSM
L
M
S
;
m
l
m
s
)
ψ
(
m
l
m
s
)
,
(1
.
2
.
14)
where the
C
(
LSM
L
M
S
;
m
l
m
s
)arethe
Clebsch-Gordan
or
Wigner
co-
ecients. It is convenient to write this expansion in a representation-





Search WWH ::

Custom Search