Biomedical Engineering Reference
In-Depth Information
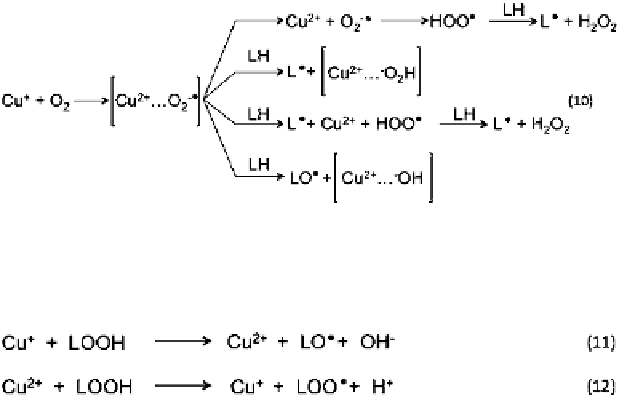
Fig. 6.15 Direct initiation of lipid oxidation by the low valence transitional metal, Cu(I). Oxygen
forms a complex with copper, permitting hydrogen abstraction and producing lipid radicals
Fig. 6.16 Hydroperoxides can be directly decomposed by copper ions, producing alkoyl radicals
(LO) and lipid peroxyl radicals (LOO), thus, propagating the lipid oxidation
lipid radicals and ROS. Resulting Cu(II) and ROS (like hydrogen peroxide) can be
“re-cycled” back to oxygen and Cu(I).
Furthermore, Cu (I) and (II) are able to propagate lipid oxidation by enhancing
the chain reaction to produce more lipid radicals [
46
,
78
]. Copper can form
complexes with lipid hydroperoxides decomposing them to lipid alkoyl radicals
(LO) and lipid peroxyl radicals (LOO) (Fig.
6.16
)[
76
,
78
].
The validity of the demonstrated reactions was confirmed in in vitro studies,
however in vivo studies still lack to show the extent of each of these reactions. Due
to the multitude of lipid oxidation reactions (Figs.
6.14
,
6.15
and
6.16
), many
intermediates can be formed capable of reacting further with formation of other
products, making these events very hard to measure and characterize in vivo.
The simple strategy used by [
39
], was to measure the concentration of a stable
lipid oxidation byproduct, such as malondialdehyde (MDA) that reacts with
thiobarbituric acid, giving an indirect quantification of lipid oxidation. Thus,
authors were able to show that lipid peroxidation occurs before cells are dead,
reaching peak in its intensity when membrane integrity is already lost [
39
].
This study demonstrated that lipid oxidation occurs by metallic copper exposure
but the exact mechanism by which copper is able to induce membrane damage still
waits to be discovered. Whether toxicity occurs indirectly through ROS or by direct
reaction between copper and lipid is still unknown. Additionally, unsaturated fatty
acids are more prone to initiation of lipid oxidation, although in bacterial mem-
branes they represent the minority of the membrane lipids. Future studies need to
address this specific mechanism of oxidation by demonstrating which lipid is
preferably targeted and if saturated fatty acids can be affected by the metallic
copper toxicity.
Search WWH ::

Custom Search