Biomedical Engineering Reference
In-Depth Information
Fig. 6.14 Direct initiation of lipid oxidation by the high valence transitional metals, such as Cu
(II). Reaction 7 illustrates an electron abstraction from a double bond; reaction 8 represents a labile
hydrogen removal. Reaction 9 is an oxidation of aldehyde group. The aliphatic chain is represented
by R
such electron spin barrier can be overcome. Copper, with its one-electron transfer
reactions, is considered an active catalyst [
85
]. Redox active metals, like copper,
are able to initiate lipid oxidation by producing lipid alkoyl radicals (LO) and lipid
peroxyl radicals (LOO) (Fig.
6.13
)[
78
]. In vitro studies were able to show that
trace metal amounts sufficed to initiate lipid oxidation [
78
,
85
], and only metals that
undergo one-electron transfers appear to be active catalysts; these include cobalt,
iron, copper, manganese, magnesium, and vanadium [
78
]. Initiation of oxidation by
redox active metals can occur indirectly by ROS or directly reaction with the lipid.
Indirect oxidation by ROS were described previously by Fenton-like reactions
(Fig.
6.2
), where the hydroxyl radical
is the main responsible for hydrogen
abstraction [
76
].
Mechanism and rate of the direct reaction between metal and lipids depend on a
multitude of factors: type of formed complex, chelator/complexing agent, redox
potential, solvent, phase localization and availability of oxygen or hydroperoxides.
Below, we will show the multiplicity of mechanisms that are possible.
The simplest mechanisms for metal catalysis is direct initiation by higher
valence metals, such as Cu(II). This implicates electron transfer from the lipid
bond to the metal. Lipid radicals (L) are formed directly by removing an electron
from the double bond (Fig.
6.14
, reaction 7), or by abstraction of labile hydrogen
from lipid molecules (Fig.
6.14
, reactions 8 and 9) [
78
].
Reactions 7 and 8 are the primarily mode of catalysis for cobalt, manganese and
chromium [
78
]. However, other metals, such as copper, can induce these reactions
when bound to a chelating agent that shifts the redox potential or in the presence of
solvents that alter acid/base properties and electron transfer efficiency. Non-polar
environments are also known to allow extremely rapid electron transfers that
generate oxidize lipids [
8
,
9
]. Oxidation of aldehydes can also occur (reaction 9)
and is strongly catalyzed by Cu(II), as well as Co(II) and Mn(II), and this reaction
occurs primarily in non-polar solvents and is inhibited by water competition [
78
].
In the case of lower valence metal ions, such as Cu(I), activation of oxygen is
required to start lipid oxidation. Cu(I) can form complexes with oxygen, thus
forming an active complex capable of attacking lipids and form lipid radicals
(Fig.
6.15
). These reactions are facilitated in hydrophobic environments [
12
].
Figure
6.15
shows multitude of ways by which Cu(I) can oxidize lipids leading to
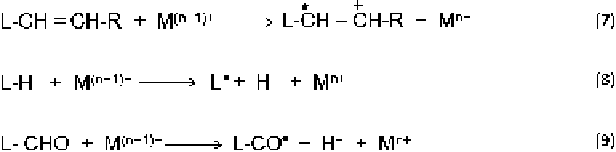
Search WWH ::

Custom Search