Biomedical Engineering Reference
In-Depth Information
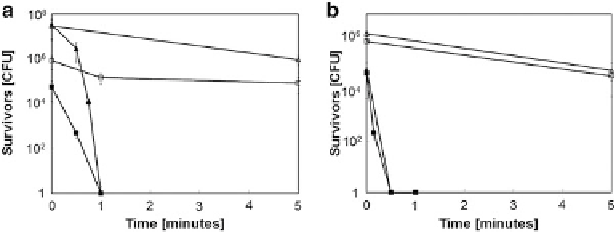
Fig. 6.12 Survival of stationary-phase (a) and exponential-phase (b) cultures of
D. radiodurans
(
squares
)or
E. coli
(
triangles
) on stainless steel (
open symbols
) or copper (
filled symbols
)
surfaces. Shown are averages and standard deviations (
error bars
) from three independent
experiments [
27
]
labels DNA of intact membrane (live) and compromised membrane (dead) bacteria.
On the other hand, propidium iodide stains only cells which have impaired mem-
branes. Thus, bacteria with intact membranes fluoresce green, while bacteria with
damaged membranes fluoresce red. Upon exposure to metallic copper, most of the
cells fluoresce red, indicating that they are all inactivated. This was the first clue
suggesting that metallic copper stress induces damage to membranes. Furthermore,
experiments performed with
S. cerevisiae
yeast cells have shown that inactivation
of cells was caused by damage inflicted on the membranes, followed by the loss of
membrane potential [
72
,
86
] and likely release of cytoplasmic contents. Further-
more, it was also observed that intracellular vesicles disappeared during exposure to
metallic copper [
72
].
Loss of respiration observed during metallic copper exposure might be another
event that supports the idea of membrane damage by metallic copper toxicity [
65
,
66
,
86
-
88
,
94
]. Such an effect can be due to loss of the proton motive force (PMF)
initiated through escape of protons through the damaged membrane. When damage
is inflicted to the membrane making it permeable, the respiratory chain becomes
uncoupled [
86
].
The investigation of this membrane damage hypothesis was further continued
by studying effect of metallic copper exposure on membrane lipid oxidation
employing thiobarbituric acid-reactive substances (TBARS) assay [
39
]. This
study delineated rapid lipid peroxidation upon cell exposure to copper, followed
by a sharp killing effect with the loss of membrane integrity at the peak level of
peroxidation. It was noted that DNA degradation only occurred after this point [
39
]
as another evidence that DNA is not the primary target of metallic copper toxicity.
Additionally, mutant strains with increased levels of unsaturated fatty acids
(
ΔfabR
), were more sensitive to copper toxicity causing TBARS levels to peak
earlier. Such observations are indicative of unsaturated fatty acids being targeted by
metallic copper toxicity. To understand how metallic copper toxicity affects
Search WWH ::

Custom Search