Environmental Engineering Reference
In-Depth Information
60
1.0
0.8
40
0.6
0.4
20
0.2
0
0.0
0
500
1000
1500
2000
Preheating temperature, K
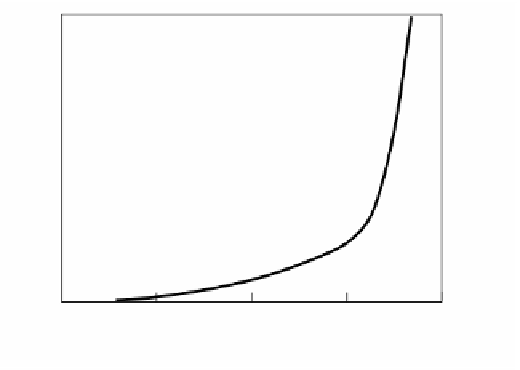
FIGURE 2.46
Burning velocity and induction period of preheated mixtures with α = 0.2095.
well-stirred reactor. This is because at 1800 K reactions begin immediately after the
mixture leaves the burner, while at 1600 K the position of the flat flame is more
dependent on the diffusion of active species and the conduction of thermal energy
to the unburned region. Especially the premature reactions at 1800 K, are fostered
by considerable amounts of CH
3
and CHO formed at the moment the mixture leaves
the burner. This means that the mixture can self-ignite and is the reason the burning
velocity increases steeply from
T
0
around 1650 K. Consequently the fuel flux also
increases: the fuel flux for the flat flame at
T
0
= 1800 K is about 17.5 times larger
than that at
T
0
= 298 K.
The imposition of preheating temperatures as high as 1800 K to mixtures is
impracticable but theoretically it would be possible to obtain stable flat flames with
burning velocities approaching 50 m/s, as shown in
Figure 2.46
.
However, the NO
after the flame front in the adiabatic model, its concentration increases monotonically
with distance. In a practical device, the temperature decreases after the flame front
and the NO would have a maximum. In a practical device, the temperature decreases
after the flame front and NO would have a maxima at a certain distance from the
flame front. Considering this fact, the calculated values at a location of 10 mm from
the flame front have been plotted as a reference NO concentration. The NO mole
fraction exceeds 2300 ppm for
T
0
= 1600 K.
2.3.2.2.2 Preheated and Diluted Premixed Flames
As seen above, preheating increases the burning velocity and consequently the fuel
flux, but large amounts of NO are formed. An ideal combustion process would be
obtained if the NO levels were lowered with the burning velocity kept large. This
can be reached by controlling the chemical reactions, which are functions of tem-
perature and the concentration of reactants. Here, to isolate the effects of temperature
and concentration on the chemical reactions, the adiabatic flame temperature
T
a
w
as
set to the fixed values of 1600, 1800, 2000, 2200, and 2400 K. Dilution ratio was







Search WWH ::

Custom Search