Environmental Engineering Reference
In-Depth Information
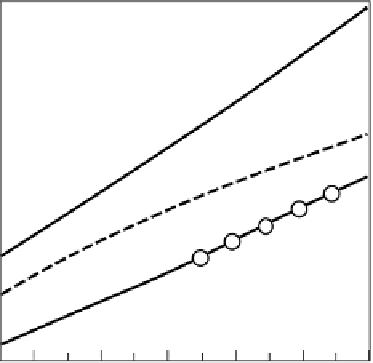
Theoretical adiabatic combustion
temperature
Adiabatic equilibrium temperature
Maximum combustion temperature
in opposed flow diffusion flame
3000
2800
2600
2400
2200
2000
400
600
800
1000
1200
1400
Preheated air temperature, K
FIGURE 3.3
Comparison of maximum temperature by different combustion models.
effect of stretch. Therefore, we can say that temperature fluctuations in a turbulent
flame may be affected not only by fluctuations in equivalence ratio but also by the
local stretch rate owing to turbulent motions.
As seen above, it is extremely difficult to predict flame temperature accurately
by numerical simulation. The only way to do this is via a simulation using the full
reaction mechanism in complex flows with a considerably short time-step, which,
however, is an unrealistic operation in engineering calculations. In the simulation
of real furnaces where time-averaged temperature and concentrations are calculated,
the only practical way is to obtain a reasonable value of combustion temperature by
correcting calculated combustion temperature, or to introduce a limit so that reactions
do not overshoot chemical equilibrium.
When reverse reactions are not included in the reaction mechanism, the com-
bustion proceeds until the concentration of one of the reactants becomes zero. But,
in reality, the chemical reaction does not go beyond chemical equilibrium, and the
real maximum flame temperature must be lower than the case where complete
reaction is assumed. The difference between the two is too large to be neglected,
especially in the high temperature range above 2000 K. For this reason we studied
a method for correcting this influence in numerical simulations where a reaction
model not including reverse reactions is used.
Temperature obtained in simulation must be corrected using the relationship
between the theoretical complete combustion temperatures and equilibrium








Search WWH ::

Custom Search