Environmental Engineering Reference
In-Depth Information
0.12
Two-dimensional Flow
T
=1280K
0.1
0.08
Yo
=0.15
Yp
=0.10
0.06
Yo
=0.07
Yp
=0.20
Cold air
0.04
0.02
Yo
=0.00
Yp
=0.30
0
1000
1500
2000
2500
Surface temperature, K
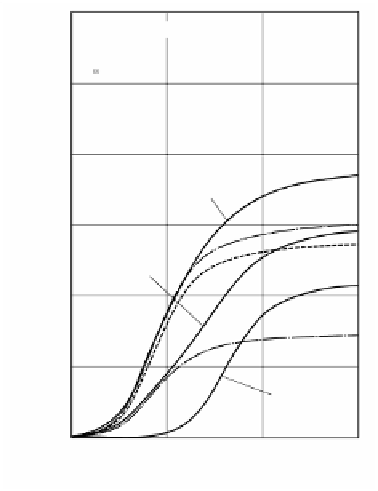
FIGURE 2.132
Influence of the concentration of the carbon dioxide in oxidizing agent flow
on the combustion rate of a solid carbon in high temperature airflow.
2.5.4.6 Lower Limit of Oxygen Concentration
With the analytical expression for the combustion rate made available, the influence
of the concentration of the oxygen in the high temperature oxidizing agent (at a
temperature of 1280 K) on the combustion rate was studied (see
Figure 2.131
)
. The
results show that a combustion rate almost equal to that available in room temperature
airflow can be obtained even if the concentration of the oxygen in high temperature
oxidizing agent is lowered to the mass rate of 0.15. In the combustion of a solid carbon,
the oxygen concentration can be further reduced in connection with the combustion
rate on the condition that the concentration of carbon dioxide is increased since carbon
dioxide can serve also as the oxidizing agent in surface reactions.
Figure 2.132
shows
the combustion rate with the densities of oxygen and carbon dioxide changed while
the mole fraction of nitrogen is kept at 0.79. It shows that, if the surface temperature
of a solid carbon is 2000 K or higher, a high temperature oxidizing agent with the
mass rates of oxygen at 0.07 and carbon dioxide at 0.20 results in a combustion rate
almost equal to that in room temperature airflow.
The relationship is expressed by the following expression:
{
}
≥
{
}
ln
1
+
β
ln
1
+
β
HO
RA
2
(2.41)
K
K
HO
RA




Search WWH ::

Custom Search