Environmental Engineering Reference
In-Depth Information
decrease at the postflame region. There are no reactions in the scheme linking indene
to larger molecules, but even with this lack of reactions consuming indene, this
species is found here to decrease through the reverse of the same reaction by which
it was produced at earlier stages. The benzene decrease is due to reactions such as
R322 and R84 and oxidation reactions like R287.
0.20
2500
CO
2000
O
2
0.15
H
2
1500
H
2
O
Temp
0.10
1000
C
2
H
4
C
3
H
8
0.05
500
CO
2
C
2
H
2
0
0
2.200
2.201
2.202
2.203
2.204
2.205
Time, s
FIGURE 2.70
Temporal profiles of temperature and main stable species around ignition at
φ = 2.
Ignition of
φ
= 2 Mixture
Next, the analysis of the ignition of a mixture of equivalence ratio equal to 2 is
described. The temporal profiles of temperature and main stable species are plotted
in
Figure 2.70
.
Here, the temperature is seen to increase monotonically with time
up to the adiabatic flame temperature, which exceeds 2180 K. According to
CHEMKIN, the ignition time is 2.2023 s. In comparison to the φ = 5 case, the
concentrations of CO
2
, CO and H
2
O are high after ignition, indicating a more
intense combustion. The concentrations of active species such as H atoms and OH
radicals are also much larger than at φ = 5: their maximum values are close to 1
and 0.2%, respectively. C
2
H
4
continues to be produced from the beginning of the
process, but its concentration reaches only 5%, lower than the 9% reached at φ =
5. The peak of C
2
H
2
is also lower, 5%. The reaction rates at this condition are
much larger; for example, R513, by which C
3
H
6
is produced from C
2
H
4
+ CH
2
,
is about 100 times faster than in the φ = 5 case. And the reaction linking C
3
H
4
to
H
2
CCCH is more than ten times larger. However, no PAH species form. Even
benzene has a peak concentration of just 1.4 × 10
-8
. C
3
H
4
and C
3
H
4
P were found
to decompose through reactions R533 and R544 just after ignition; H
2
CCCH keeps
a significant concentration at later stages, but the rate of the step H
2
CCCH + C
2
H
2
→ C
5
H
5
(L) (R431) is about 100 times smaller than at φ = 5. The rate coefficient
of this reaction is expressed as 5.62 × 10
32
T
-7.3
e
xp(-6758cal/mol)/
RT
), as shown

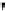

Search WWH ::

Custom Search