Environmental Engineering Reference
In-Depth Information
2.0
A3
1.6
FLTHN
1.2
A2YNEP
0.8
COR
C
10
H
7
OH
C
12
H
10
ACEPHA
30
0.4
0
0
5
10
15
20
Time, s
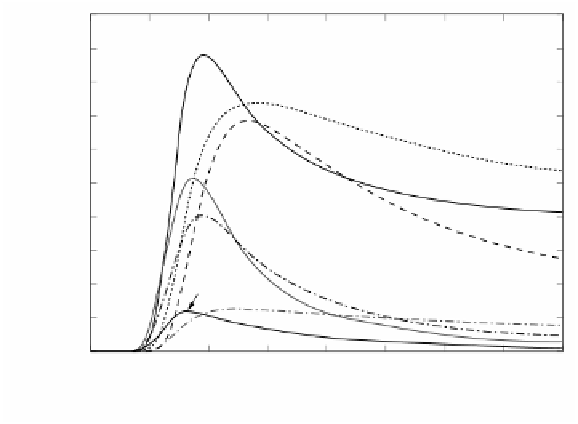
FIGURE 2.68
Temporal profiles of species containing two, three, and more aromatic rings
at φ = 5.
cyclic (C
5
H
5
, cyclopentadienyl radical) through M R430. C
5
H
5
reacts with C
2
H
2
to
form C
7
H
7
(radical of toluene) through R380. Further addition of C
2
H
2
to the latter
species forms INDENE (R10). Indene is formed up to 1.5 s after ignition, but later
R10 proceeds in the reverse direction, and indene decomposes back into C
7
H
7
+ C
2
H
2
.
The time at which R10 changes sign coincides with that at which the above
cited reactions R532, R431, R430, and R380 peak. This time interval of about 2 s
after the first stage can be referred to as the second stage, where indene also peaks,
as can be seen in
Figure 2.67
.
Indene can form indenyl radical (INDENE*) through
H abstraction, but because it does not participate in reactions leading to larger PAH
molecules, its concentration is largely defined by R10 only, having a maxima close
to 2%. Yet, in the second stage, two routes of decomposition of C
7
H
7
are observed:
one into C
6
H
5
(phenyl radical) through -R378 and the other into C
6
H
6
after formation
of C
7
H
8
(toluene) (-R367, R368).
From the third stage, in which the reactions peak about 1 s later than the reactions
of the second stage, larger aromatic species form almost exclusively through HACA
(hydrogen abstraction, C
2
H
2
addition) reactions. From C
6
H
6
, H abstraction (R383)
and C
2
H
2
addition (R358) give C
8
H
6
(phenyl acetylene). From C
8
H
6
, H abstraction
(R340) and C
2
H
2
addition (R343) form C
10
H
7
S (1-naphthyl radical). H addition
through reaction of H
2
with C
10
H
7
S (R336) gives C
10
H
8
(naphthalene). C
2
H
2
addition
into 1-naphthyl (R19) forms A2R5 (acenaphthalene).
From naphthalene, H abstraction (R337) gives C
10
H
7
P (2-naphthyl radical) and
C
2
H
2
addition (R67) to the latter species leads to A2YNEP (C
12
H
8
, 2-naphthylacet-
ylene). Naphthalene can also be oxidized via reaction with OH radicals (-R13) with
to A3*S1 (1-, 8-, 9-, and 10-phenanthryl radical). Further C
2
addition (R82) forms
Search WWH ::

Custom Search