Biology Reference
In-Depth Information
To better understand the following discussion, let us
refresh our memories with the most simplified model of the
core clock. In this model, interlocking feedback loops of
transactivators (Bmal1; Clock/Npas2) and transrepressors
[Per(1,2); Cry(1,2)] are regulated at
clocks are susceptible to entrainment by a wide variety of
environmental cues, such as temperature and fasting/
feeding schedules. The 24-hour period of the clock is found
to be resistant to temperature fluctuations within the bio-
logical range (i.e., clocks are temperature compensated)
[194]
, but unlike in the SCN, changes in the ambient
temperature cause re-entrainment or phase resetting of the
peripheral clocks
[195]
. Also, gene-expression rhythms in
the peripheral clocks of Cry1
-/-
/Cry2
-/-
and SCN lesioned
mice are restored in a restricted feeding paradigm, indi-
cating that even in the absence of the central clock, feeding
schedule is functioning as a zeitgeber (time cue) for the
peripheral clocks
[196
the transcriptional
level by nuclear hormone receptors
RORs (Rora, Rorb,
Rorc) and REV-ERBs (Nr1d1 and Nr1d2) and at the post-
translational level by casein kinase 1-
d
/
e
)to
produce rhythmic output with a 24-hour period (see
Figure 21.2
). Peripheral clocks harbor this core-clock
machinery of the SCN but with some interesting (and many
speculative) differences. In the SCN, the Clock gene is
functionally compensated by Npas2
[189]
, but is indis-
pensable in peripheral tissues
[190]
. This suggests tissue-
specific differences in the molecular make-up of the clock
network. This hypothesis is strengthened by recent studies
wherein tissue-specific disruption of clock in the liver
[191]
and pancreas
[192]
gave mirror-opposite phenotypes.
In addition to these intracellular differences, strong
intercellular coupling is a unique property of the SCN.
Three well-defined modes of cell
(CSK1-
d
/
ε
ε
198]
. In summary, all clocks use
the same building blocks, but the network properties and
mechanistic details of peripheral clocks are probably
different from that of the SCN
[199]
.
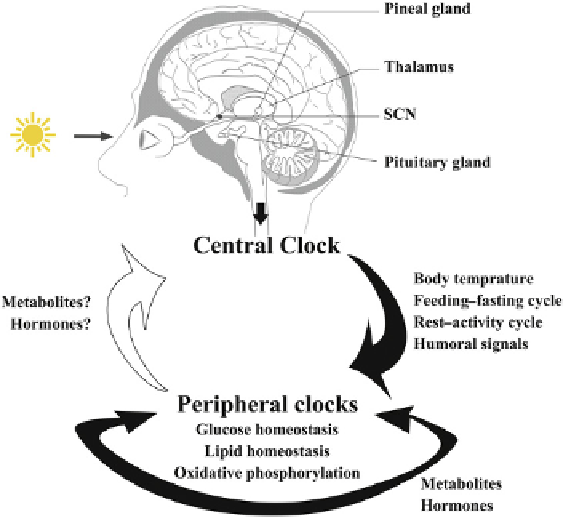
Causality in inter-clock communication is the subject of
intense investigation. The SCN can communicate with the
other clock-containing regions (directly or indirectly) by
neuronal innervation or by long-range humoral signals
such as glucocorticoid hormones (
Figure 21.3
;
[27,200]
).
Retrograde and anterograde labeling have been used to map
direct neuronal innervation from the SCN to the cell bodies
in the neighboring hypothalamic nuclei, for example the
arcuate nucleus (ARC), periventricular nucleus (PVN),
lateral hypothalamic area (LHA) and dorsomedial hypo-
thalamic area (DMH) (see
Figure 21.1
;
[201,202]
). The
ARC is a well-known center for the synthesis and secretion
of neuropeptides that can either increase appetite (orexi-
genic), namely neuropeptide Y/Agouti-related protein
e
cell communication
exist in the SCN: gap junctions, peptidergic signaling using
the neuropeptide vasointestinal peptide (VIP) and its
cognate VPAC2 receptor, and GABA signaling
[27]
.
Rhythms of peripheral organs ex vivo dampen in the
absence of the intercellular coupled architecture seen in the
SCN. Hence, we can envision that in vivo peripheral
oscillators require synchronization cues potentially from
the SCN (and/or other yet unrecognized master oscillator(s)
[193]
). This hypothesis is demonstrable as peripheral
e
FIGURE 21.3
A schematic representation of cross-talk
between the central and peripheral clocks. These communica-
tions are known to be governed by environmental, physiological
and metabolic cues. The nature and extent of this inter-clock
communication are subjects of intense discussion (see text). An
increasing pool of research is identifying the causal role of
peripheral clocks in regulating various metabolic processes, and
the dysregulation of this interplay is found to be an etiological
factor for human diseases such as obesity and diabetes.