Biology Reference
In-Depth Information
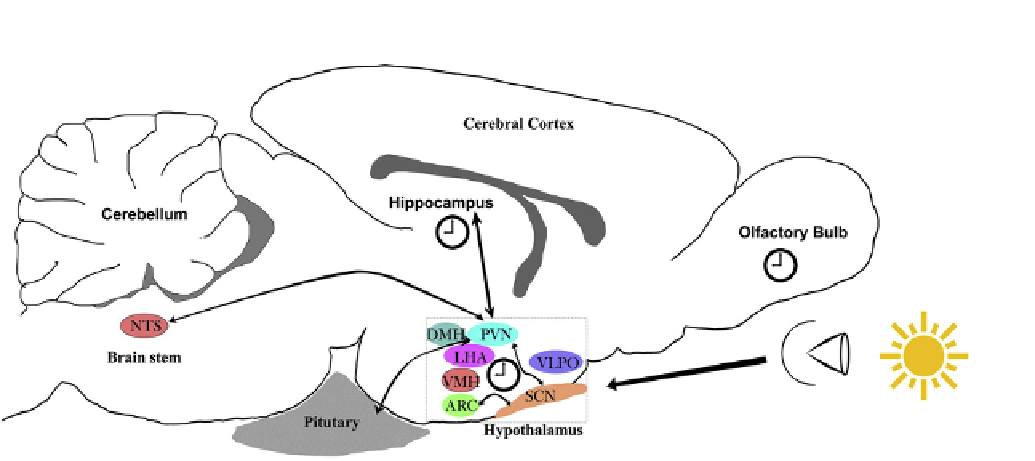
FIGURE 21.1
Inter-clock communication within the brain. A schematic sagittal view of the mouse brain with the master clock, the suprachiasmatic
nucleus (SCN) positioned in the anterior hypothalamus. This small cluster of ~50 000 neurons in the SCN receive photic input from the retina and are able
to autonomously generate circadian oscillation in phase with the environment. Efferent processes from the SCN innervate neighboring nuclei such as the
arcuate nucleus (ARC), paraventricular nucleus (PVN) and lateral hypothalamus (LHA) which regulate a plethora of homeostatic processes. Furthermore,
the PVN functions as a relay center and connects with numerous other regions, such as the hippocampus of the temporal lobe, the nucleus of the solitary
tract (NTS) in the brainstem and the pituitary gland
e
the basecamp for all major regulatory hormones. In addition to the SCN, various other oscillators
have been discovered within the brain, e.g., the olfactory bulb in rodents, the ARC nucleus and more recently, the hippocampus, the center for learning and
memory. DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus; VLPO, ventrolateral preoptic nucleus. Figure not drawn to scale.
circadian pacemaker in mammals. The master clock in
mammals is a cluster of ~50 000 neurons placed anteriorly
to the optic chiasm and hence referred to as the supra-
chiasmatic nucleus (SCN;
Figure 21.1
). The SCN receives
sensory light inputs from the retina, and in turn the SCN
connects with structures within the brain and in peripheral
organs (
Figure 21.1
; also discussed further below). The
SCN is able to generate autonomous and sustained circa-
dian oscillations that can be observed at the electrophysi-
ological, behavioral and molecular levels
[5,27]
.
First, let us define sleep, which differs depending on the
organism studied and how it is evaluated
[30,31]
. Sleep can
be scored qualitatively based on behavioral parameters
such as a period of inactivity or poor responsiveness to
external stimuli, and quantitatively by physiological
parameters such as brain electrical activity (at least in
higher vertebrates such as birds and mammals). In the
absence of electrophysiological signs, reptiles, amphibians,
fish, and invertebrates are said to undergo 'rest' resembling
that of higher vertebrates (with some exceptions: 30,31).
Classically, studies of human sleep disorders were carried
out using electroencephalographic (EEG) recordings that
identified two major components in sleep architecture.
These are named after their phenotypic correlates in eye
movement, rapid eye movement (REM) sleep and non-
REM (NREM) sleep
[32]
. During waking, brain waves are
observed to be high-frequency low-amplitudes spikes that
are believed to arise from the internal desynchrony of the
active cortical neurons
[33]
. With progression of sleep into
REM and subsequently to the more stable and deeper
NREM sleep, EEG recordings
Sleep
The influence of the biological clock is most obvious in the
timing of sleep. In diurnal organisms, behavioral and
cognitive performance peak during the subjective day,
while the opposite occurs in nocturnal animals. However, in
both diurnal and nocturnal animals performance is also
associated anecdotally with sleep/wake history, i.e.,
a sleep-deprived individual will perform poorly compared
to a fully rested individual
[28]
. Such observations suggest
an obvious link between the circadian system and the
sleep
tend towards
lower-
frequency (
75
m
V; also
termed
d
waves) potentially arising from residual but
synchronized neuronal activity
[33,34]
. In humans NREM
is further subdivided into the N1, N2 and N3 stages. The N3
stage (also called slow-wave sleep (SWS)) represents the
deepest form of sleep, where 20% or more of all recorded
signals observed within a 30 s window (an epoch) are
d
waves
[32,35]
. The prevalence and amplitude of EEG
2 Hz) high-amplitude waves (
<
>
wake cycle. But why do we sleep? Numerous
hypotheses have been proposed, and this topic remains
contentious. A recent hypothesis by Tononi and colleagues
has gained much popularity
[29]
. They suggest that sleep
provides a mechanism to reboot neuronal activity and
return it to baseline (energetically) after a day of interaction
with the physical world
[29]
.
e