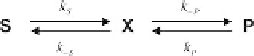Biology Reference
In-Depth Information
work of Cotterell and Sharpe
[38]
, which suggests that it
may be possible to start with a large class of models capable
of capturing well-established features for a developmental
system and systematically reduce this class to a signifi-
cantly smaller set of models that improve the identification
of candidate hypotheses for testing. The second task,
assuming that an explicit model or suite of models is in
hand, is also a daunting problem. Exploring the full
phenotypic potential of even a relatively small non-linear
model, say with 15 parameters, by analytical methods is
intractable. Furthermore, an empirical sampling of alter-
natives suffers from a combinatorial explosion. (If one were
to sample just 10 values of each parameter in all combi-
nations it would require 10
15
simulations, and one might
still have missed some important behavior beyond the
sampled range, or between sampled points.) We have
recently provided a generic definition of 'phenotype' based
on combinations of dominant processes operating within
a system
[39]
. We showed how this definition partitions
a 'system design space' into qualitatively distinct pheno-
types with rigorously defined boundaries. With this
approach, phenotypes are identified and enumerated, their
relative fitness is compared, and their tolerance to pheno-
typic change measured.
Phenotypes from the Analytical Solution
In fact, we can solve for its behavior, since it is a simple
linear system. The differential equation governing this
system is the following.
dX
dt
¼
k
s
S
k
s
X
k
p
X
k
p
P
(1)
Setting the derivative to zero and solving for the dependent
variable yields the steady-state solution
k
S
S
k
P
P
k
S
þ
k
P
þ
X
¼
(2)
or, since detailed balance requires
k
S
k
S
¼
k
k
S
k
P
k
P
¼
P
k
S
k
P
¼
K
eq
;
K
eq
K
eq
K
eq
¼
and
K
eq
Equation
(2)
can be rewritten as
ð
k
S
=
k
P
Þ
K
eq
S
þ
P
=
K
eq
ð
k
S
=
k
P
Þþ
X
¼
(3)
1
Thus, the solution is characterized by two equilibrium
constants (which are fixed thermodynamic quantities), two
kinetic parameters (which are subject to change with the
design of a catalyst), and the two independent concentra-
tion variables (which are subject to direct experimental
manipulation of the environment).
Examination of the solution in Eq.
(3)
suggests four
qualitatively distinct phenotypes based on the dominance
of terms in its numerator and denominator:
Case 1
:
X
z
K
eq
S
when
k
S
k
Generic Concept of Phenotype
We define a qualitatively distinct phenotype as the set of
concentrations and fluxes corresponding to a valid
combination of dominant processes functioning within
a system. Each of the terms in this definition requires
further explanation for this definition to be useful. We do
this first in the context of a very simple system and then
show how this definition applies to more complex
systems. With this approach in mind, we start with a two-
step series of elementary chemical reactions as shown
in
Figure 15.3
. For example, this could represent the
reactions involving the acyclic form of D-glucose that
exists during transitions between the alternative cyclic
forms
[40]
.
What is the repertoire of qualitatively distinct pheno-
types for this simple system? If we could analytically solve
for its behavior we might be able to identify distinct
operating regimes from the structure of the solution.
1
K
eq
G
k
S
k
P
>
and
P
>
1
K
eq
S
z
ð
k
S
=
k
P
Þ
Case 2
:
X
when
k
S
k
1
K
eq
G
k
S
k
P
>
and
P
<
1
K
eq
Case 3
X
z
ð
k
P
=
k
S
Þ
P
=
:
when
k
S
k
1
K
eq
G
k
S
k
P
<
and
P
>
1
K
eq
Case 4
X
P
=
:
z
when
k
S
k
1
K
eq
G
k
S
k
P
<
and
P
<
1
where the conditions can all be expressed in terms of the
genetically influenced and independent kinetic parameters
for the mechanism (
FIGURE 15.3
Two-step pathway of elementary first-order chemical
reactions. The concentrations of the initial substrate, S, and the final
product, P, are independent variables representing a fixed environment.
The concentration of the intermediate, X, is the single dependent variable
in this system. The rate constants k
i
and k
i
are constrained by the equi-
librium constants of the reversible reactions.
), the environmental influ-
enced and independent variables (
G ¼
S
=
P), and the
overall thermodynamic constant (K
eq
).
ð
k
S
=
k
P
Þ