Biology Reference
In-Depth Information
determined by the activity of specific E3 ubiquitin-ligating
complexes (APC and SCF)
[29]
. Polyubiquitinated cyclin
molecules are rapidly degraded by proteasomes in the cell.
Specific CDK:cyclin heterodimers are active at distinct
phases of the cell cycle (
Figure 14.3
). In early G1 phase, cells
are mostly devoid of cyclin molecules, except for minor
amounts of cyclinD in combinationwith either Cdk4 or Cdk6
[28,30]
. In late G1, cyclin E makes a brief appearance when,
in combination with Cdk2, it turns on the transcription factor
for cyclin A and turns off the ubiquitination of cyclin A
[31]
.
Hence, cyclinA accumulates and, in combinationwith Cdk2,
drives the cell through S phase. InG2 phase, cyclinAchanges
partners to Cdk1 and promotes the production of cyclin B.
Cdk1:CycB heterodimers are essential for successful
completion ofmitosis. During prometaphasemost cyclinA is
degraded, but cyclin B persists at high levels right up to
metaphase
[32]
. During metaphase and anaphase, cyclin B is
rapidly cleared from the cell, leaving the daughter cells in G1
phase with only the remnant supply of cyclin D.
There are two other modes of CDK regulation that are
crucial for cell cycle control (
Figure 14.2
). First, both Cdk1
and Cdk2 can be phosphorylated on neighboring threonine
and tyrosine residues in the N-terminus of the polypeptide
chain
[33]
. These phosphorylations, which significantly
inhibit the activity of the CDK:cyclin heterodimer, are
carried out by members of the Wee1 family of protein
kinases. To regain catalytic activity, the heterodimer must
be dephosphorylated by a member of the Cdc25 family of
protein phosphatases
[34]
. How Wee1 and Cdc25 activities
are regulated will be described later. Second, there exist
families of cyclin-dependent kinase inhibitors (CKIs) that
bind strongly to CDK:cyclin dimers to form inactive
trimers
[35]
. The fraction of the CDK:cyclin pool that can
be inhibited in this way depends on the abundance of CKI
molecules. Like cyclins, the abundance of a CKI is deter-
mined by its rates of synthesis and degradation
[36]
.
A few other molecular components deserve special
attention (see
Table 14.2
for a summary). Cyclin D, cyclin
E and CKIs are ubiquitinated by an E3 ubiquitin ligase
called SCF, which recognizes its substrates only when they
are properly phosphorylated. Hence, the degradation of
these regulatory proteins can be controlled by specific
protein kinases. Cyclins A and B are ubiquitinated by the
'anaphase-promoting complex/cyclosome' (APC/C),
which requires an auxiliary protein (Cdc20 or Cdh1) to
target specific substrates to the APC. The phosphorylation
states of the APC and its binding partners, Cdc20 and Cdh1,
determine the activity of the complex
[37]
. In pro-
metaphase APC:Cdc20 actively degrades cyclin A but not
cyclin B. Cyclin B degradation is delayed until late meta-
phase
[38]
, concurrently with securin (next paragraph).
Cohesin rings are cleaved by a protease called separase,
which is kept inactive throughout most of the cell cycle by
being bound to an inhibitor called securin
[39]
. During
prometaphase, as the replicated chromosomes are being
aligned on the mitotic spindle, the activity of APC:Cdc20
toward securin and CycB is blocked by an inhibitor, Mad2
[40]
. When all chromosomes are properly aligned, the
mitotic checkpoint is lifted and Mad2 is removed from
APC:Cdc20, which then ubiquitinates securin, leading to
its degradation by proteasomes. Free molecules of separase
then cleave cohesin rings and promote anaphase.
The concurrent degradation of CycB during late meta-
phase and anaphase helps the cell to return to the G1 state.
In budding yeast cells, as we describe later, the return to G1
is aided by the activation of Cdc14 (a Cdk counter-acting
phosphatase) and Cdh1 (a Cdc20-homolog)
[41]
.
From this brief description of the molecules that control
cell cycle progression some specific features are incredibly
obvious, such as the roles of CDKs in triggering DNA
synthesis and mitosis, or the role of APC in promoting
anaphase, but the subtle details of cell cycle control remain
shrouded in mysteries. To understand exactly how the four
fundamental properties of cell cycle progression are
ensured by the underlying cell cycle machinery we must
address the problem from a 'systems' point-of-view, asking
two main questions. What are the basic principles of cell
cycle regulation? And how are these principles imple-
mented in molecular interactions? If we can answer these
questions satisfactorily, then the whole welter of facts and
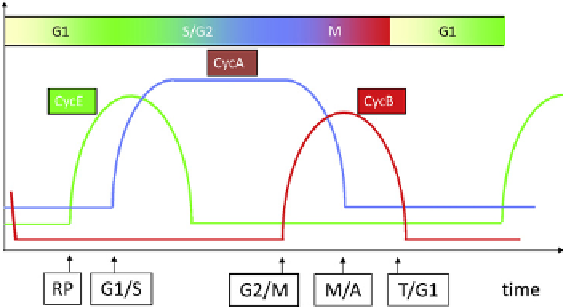
FIGURE 14.3
The cell cycle of a 'generic' eukaryote. We track
fluctuations in three major classes of cyclins (A, B and E). In early
G1 phase, all three classes of cyclins are absent. In mid-G1, CycE
begins to rise at an event called the restriction point (RP) in
mammalian cells (Start in yeast cells). CycA-dependent kinase is
responsible for initiating DNA synthesis, so it rises at the G1/S
transition. CycB-dependent kinase is responsible for mitosis, so its
activity rises at the G2/M transition. CycA level falls in prom-
etaphase, but CycB level falls later (after chromosome alignment
on the metaphase plate).