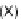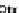Biology Reference
In-Depth Information
(A)
(B)
(C)
FIGURE 11.4
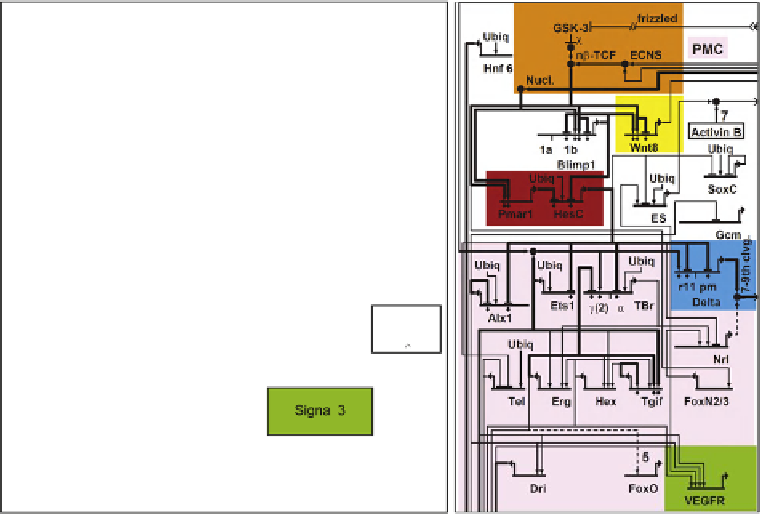
Network circuitry underlying early spatial inputs in the sea urchin embryo. (A) Color-coded diagram showing information flow
that functions to provide spatial organization in the early embryo. Each colored box includes specific GRN circuitry, including emission and reception of
signals. The location in the embryo where the respective GRN circuitries are active are shown by the red areas of the diagrammatic embryos. Where signal
interactions are depicted, arrows lead from the source of the signal to the target cells. In the skeletogenic spatial circuit, X indicates the domain where the
skeletogenic GRN is installed, 1-X the remainder of the embryo where it is repressed. (B) Lateral diagram of blastula stage embryo showing relative
spatial disposition of skeletogenic, mesodermal and endodermal precursors (p.). (C) Skeletogenic GRN shown in detail with circuitry highlighted
according to the color code of (A).
expression in a specific compartment but at the same time
ensures that these same genes are repressed in all other
cells. Especially in early developmental stages, when
most cells are not yet specified, such binary regulatory
outcome (X: where the skeletogenic genes are expressed,
1-X: the rest of the embryo) combined with repression
provides a frequently used cell fate exclusion mecha-
nism, which in one or the other of its states functions
globally
[21]
.
transcriptional regulators in these adjacent cells which are
thereby specified as mesoderm
[54,68]
.
The specification of endodermal cell fates depends on
another signaling interaction originating in the skeleto-
genic cells. The skeletogenic GRN causes expression of
Wnt signaling ligands (
Figure 11.4
A, 'Signal 2') which
diffuse to neighboring cells where, in consequence, an
endodermal GRN is activated
[15,53,69,70]
.Mu iple
transcription factors in the GRN specifying endodermal
fates are directly regulated by Tcf, which is the early
response factor for Wnt signaling
[52,53,55]
. The Delta/
Notch and Wnt signaling interactions globally control
spatial expression of their respective endodermal and
mesodermal target genes globally
[53]
. An interesting
aspect of this circuitry is that both early response factors,
Tcf (Wnt signaling) and Su(H) (Delta/Notch signaling)
associate with the co-repressor Groucho to mediate
repression of their target genes in cells not receiving the
corresponding signaling ligand. Thereby, these target
genes are activated in cells receiving the signal and
repressed in all other cells, mediating an X/1-X regulatory
logic similar to the one used to initiate the skeletogenic
GRN though using different circuitry.
The skeletogenic cells are not dependent on any
signaling input as long as they remain localized at the
vegetal pole. But as soon as these cells start to migrate into
Example 3: Signaling and Cell Fate
Specification
GRNs driving the specification of individual embryonic
domains must communicate with each other to ensure
correct spatial organization. Skeletogenic cells do not
require any signaling inputs from the cells surrounding
them to accomplish their own specification, but they do
function as a signaling center for adjacent cells. That is, the
skeletogenic GRN controls expression of genes encoding
signal ligands. Downstream of the double-negative gate,
under direct control of repressor HesC, is the gene encoding
the signaling ligand Delta (
Figure 11.4
A,C 'Signal 1')
[65
67]
. Delta is a membrane-bound signaling ligand
which activates the Notch receptor on immediately adja-
cent cells. The consequence is to activate a new network of
e