Environmental Engineering Reference
In-Depth Information
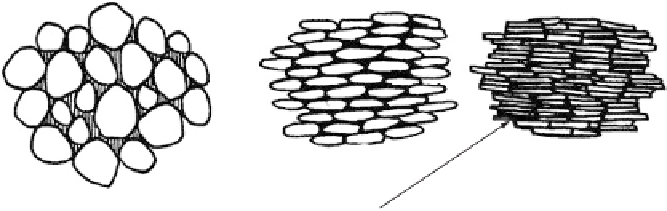
Sand
Silt
Clay
Pore space between particles occupied by water or air
Fig. 3.5. Total porosity of clay is much larger than that of sand, but the pores may be
too small to benefit plants. (Drawing by R. Castro.)
the other hand, soil with large pores and weak capillary forces may hold
only a small percentage of water, allowing the rest to drain by gravity
before it can be absorbed by roots (Fig. 3.6). Not only is this water lost, but
it may carry dissolved nutrients away as well.
Soil Cation Exchange Capacity
During physical and chemical weathering, soil minerals and organic
matter break up into very small particles called colloids. These particles
are generally negatively charged and can therefore attract and hold
positively charged ions, called cations. Many plant nutrients, such as
potassium (K
þ
), ammonium (NH
4
), calcium (Ca
2
þ
) and magnesium
(Mg
2
þ
) exist in soil solution as cations and are attracted by negatively
charged colloids. The cations are held in what is called exchangeable
form; i.e. they can be exchanged with other cations and become available
Air
Water drains slowly,
and a large amount
of it remains in the
root zone
Water drains
rapidly, and little of
it remains in the
root zone
Fig. 3.6. In more porous soil, the capillary forces holding water are weaker, and it
may drain away before roots can absorb it. (Drawing by R. Castro.)