Civil Engineering Reference
In-Depth Information
HPSCC,400
HPSCC,400,MS10%
HPSCC,400,NS
2%
HPSCC,400,NS
2%MS10%
HPSCC,450
HPSCC,450,MS10%
HPSCC,450,NS
2%
HPSCC,450,NS
2%MS10%
HPSCC,500
HPSCC,500,MS10%
HPSCC,500,NS
2%
HPSCC,500,NS
2%MS10%
720
620
520
420
320
220
120
20
0
10
20
30
40
50
60
70
80
90
100
Time (days)
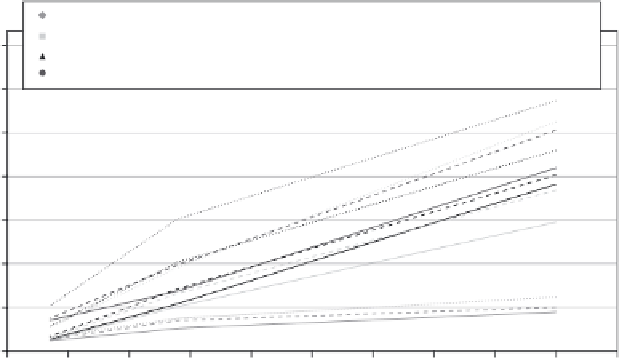
3.3
Resistivity versus time for different mixtures (Nazari and Riahi,
2011b).
between nanoparticles existing in higher concentration, so limiting the for-
mation and growth of Ca(OH)
2
crystals due to space limitations. In this
situation, the ratio of crystals to C-S-H gel is reduced and the shrinkage
and creep of the cement matrix tend to increase. In consequence, the pore
structure of the cement matrix is relatively more coarse (Zhang and Li,
2011).
3.2.3 Control of calcium leaching
High durability concrete requires the reduction of calcium leaching. This
degradation process consists of a progressive dissolution of the cement
paste caused by the migration of calcium atoms to the aggressive solution.
Cement paste phases have different rates of degradation. While Portlandite
dissolves completely in an aggressive solution, C-S-H gel undergoes only a
slight increase in porosity (Carde
et al.
, 1996; Kamali
et al.
, 2003; Haga
et al.
, 2005; Gaitero
et al.
, 2012). Calcium leaching is responsible for an
increase in concrete porosity and consequently in increased permeability.
This allows water and other aggressive elements to enter the concrete which
causes carbonation and corrosion problems. Gaitero
et al.
(2008) studied
the infl uence of silica nanoparticles on the reduction of calcium leaching.
Concrete mixtures containing 6% (by weight of cement) of four different
types of commercial silica nanoparticles (Table 3.1) were used.
Figure 3.4 shows that the addition of silica nanoparticles to the cement
paste favors the growth of silicate chains. This is advantageous as longer




















Search WWH ::

Custom Search