Civil Engineering Reference
In-Depth Information
2
3
7
8
9
10
1
12
6
13
4
mA
A
11
V
5
220V
16.5
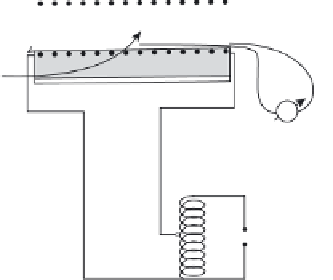
Schematic of the spray pyrolysis setup: (1) pyrex sprayer,
(2) container for ferrocene-benzene solution, (3) peristaltic pump,
(4) nitrogen gas fl ow meter, (5) nitrogen gas cylinder, (6) quartz
template, (7) furnace outer shell, (8) thermal and electrical insulation,
(9) heating element, (10) quartz tube, (11) power supply,
(12) thermocouple, (13) outlet to exhaust (adapted with permission
from Dasgupta
et al.
, 2008).
A noteworthy breakthrough in the area of development of nanotube
chemistry is the oxidation of CNT in concentrated nitric acid (Rosca
et al.
,
2005). Such a drastic condition helps in opening of the CNT tips as well as
oxidative etching along the sidewalls enabling the decoration of walls with
various oxygen-containing groups (mainly carboxyl group). The incorpora-
tion of carboxyl group exposes various useful sites in CNTs for further
modifi cation as per requirements (ester or amide bond formations can take
place). In addition, the formation of anhydride at the tube ends can take
place through which the rings of CNTs are accessible (Sano
et al.
, 2001).
The most important implication of the introduction of a carboxyl group lies
in the fact that the van der Waals forces existing between the individual
CNTs are reduced and hence the CNTs can be made water soluble (as-
grown CNTs are not soluble in any solvent) by addition/substitution of new
moieties.
On the other hand, addition reactions help in direct coupling of func-
tional groups onto the
π
-conjugated carbon framework. A series of addition





















Search WWH ::

Custom Search