Civil Engineering Reference
In-Depth Information
surfaces.
54
Wang
et al.
have reported that the transition between the hydro-
phobic and hydrophilic states could possibly be connected to photoactive
electronic transition across the energy gap, i.e., the conversion of Ti
4+
sites
into Ti
3+
on the surface under UV illumination.
59,60
Therefore in terms of
UV activation, there are common features between the photocatalytic
mechanism and hydrophilicity.
61
Recently, however, there has been some consensus that the basic mecha-
nism of these two phenomena may not be the same. According to Watanabe
et al.
,
62
the existence of sodium ions in TiO
2
showed very different effects
on these photoinduced reactions, suggesting two different photoinduced
defect reaction mechanisms on the surface. The essential photocatalytic
mechanism could be explained in terms of bulk properties, such as the
charge transfer effi ciency of a wide gap semiconductor. Therefore it seems
photocatalysis of TiO
2
is more dependent on bulk properties, while the
hydrophilicity of TiO
2
is an inherently interfacial property, limited to the
interface between TiO
2
surface (solid) and water (liquid).
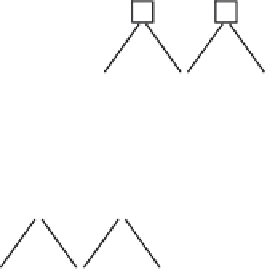
The hydrophilic mechanism is believed to be as follows; electrons reduce
the Ti (IV) cations to the Ti (III) state, and the holes oxidize the O
2−
anions.
In the process, oxygen atoms are ejected and oxygen vacancies are created
(Fig. 14.2). Water molecules can then occupy these oxygen vacancies, pro-
ducing adsorbed OH groups, which tend to make the surface hydrophilic.
63
14.3.2 Anti-bacterial action
There are two principal ways to realize self-cleaning material surfaces: the
development of superhydrophobic or superhydrophilic materials. By trans-
Oxygen vacancies
Ti
Ti
UV
Dark
H
H
O
O
O
O
Ti
Ti
Ti
Ti
Ti
Ti
Hydrophobic
Hydrophilic
14.2
Mechanism of photo-induced hydrophilicity.
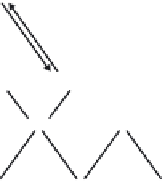
Search WWH ::

Custom Search