Civil Engineering Reference
In-Depth Information
NO
2
PM
NO
2
PM
PM
NO
2
MA
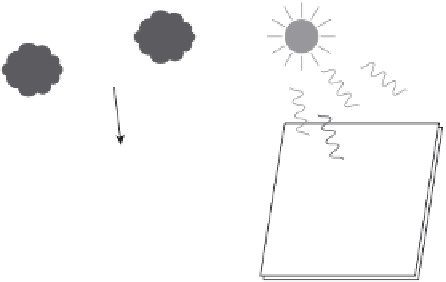
Draining
13.5
Schematic representation of self-cleaning on TiO
2
containing
surfaces: PM
=
particulate matter, MA
=
mineral acids.
contact angle lower than 20°, as in the case of irradiated TiO
2
(Fujishima
and Zhang, 2006).
To be precise, this property of self-cleaning should be referred to as 'easy
cleaning': in fact, dirt and particles can adhere on the surface of titanium
dioxide, even when irradiated; it is then extremely easy to remove them, as
only UV light (available in natural sunlight) and water (that can be pro-
vided by rain) are required to remove stains and dirt.
Self-cleaning has become popular in a number of fi elds, even in clothing:
some companies are producing self-cleaning cotton fabric, for an easier
cleaning and deodorizing of clothes. Yet, their chief success is in the built
environment, with the production of self-cleaning glass, tiles, paints, mortars
and many other materials and components. In building façades, soiling is
due to the adhesion of particulate matter on the surface of the materials
they are made of, which are often porous (and therefore more prone to
adsorbing such compounds). Particulate usually attaches to the surface
through organic bonds, such as fatty acid chains and carboxylic groups. The
twofold role of TiO
2
is then the photocatalytic degradation of such groups
and the onset of superhydrophilicity, which drives rainwater in direct
contact with the surface, removing particles which are at that point loosely
adherent, thus reducing the need for maintenance.
13.3.3 Antibacterial and anti-vegetative properties
Another possible way of exploiting the photocatalytic properties of TiO
2
is
its ability to mediate the destruction of bacteria, viruses and other biological
materials. This function is often referred to as photosterilization, and it is
of great interest for applications connected to air depuration - possibly of













Search WWH ::

Custom Search