Environmental Engineering Reference
In-Depth Information
Phenol
2-Methylquinoline
Benzene
Benzofuran
Toluene
Benzothiophene
100
10
1
0.001
0.01
0.1
1
10
c
/mg L
-1
FIGURE 11.7
The experimentally determined mixture loadings (symbols) of a mixture consisting of six com-
ponents on AC F200 (Chemviron) at
T
= 20 ± 3°C in water and a nonlinear global fit (lines)
using the IAS-model.
(phenol or benzene) is typical for all activated carbons in the concentration
range investigated.
The experimental data points of multiadsorption isotherms were evalu-
ated by the IAS-model using the Freundlich isotherm as input parameters. If
all parameters for all compounds (
K
and
n
) are known, the IAS-model esti-
mates the loads of the corresponding equilibrium concentrations.
For
n
compounds, a fit with 2
n
parameters (
K
and
n
) is necessary, for
example, for six compounds, 12 parameters have to be fitted. However, two
parameters are always connected with the experimental data (equilibrium
concentrations and corresponding equilibrium loads) for each isotherm. In
total, 60 data points are typically available. The criteria in the fit are defined
in Equations 11.6 and 11.7:
60
∑
(
χ
2
2
=
q
−
q
)
→
minimum
(11.6)
IASi
,
eq i
,
i
=
1
q
=
f(K nc ) for allcompounds
,,
(11.7)
IASi
,
eq
where c
eq,i
and q
eq,i
denote the experimental data and q
IAS,i
denotes the fitted
load from the IAS-model for compound i.
The load obtained by the IAS-model q
IAS,i
depends on Freundlich param-
eters
K
and
n
for all compounds, and parameters are varied until the mini-
mum value for χ
2
is found. The lines shown in Figure 11.7 are the result of
such a fit obtained for the activated carbon F200.




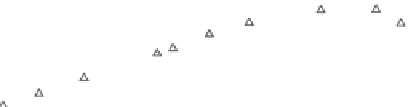


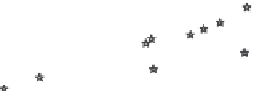






Search WWH ::

Custom Search