Environmental Engineering Reference
In-Depth Information
heterogeneous spectrum of compounds typically present in contaminated
groundwater. In addition, pK
a
-values of many N-HETs and especially
hydroxylated N-HETs in the range of 3 < pK
a
< 7 suggest pH-dependent
properties. This is an important factor that needs to be taken into account
in designing analytical methods and adsorption experiments. Due to pH-
dependent adsorption parameters, the adsorption equilibrium could be
different for cations (e.g., N-HETs at pH ≪ pK
a
) and anions (e.g., phenols or
carboxylated compounds at pH ≫ pK
a
) compared to the neutral molecules
[11-15]. Therefore, pH-values at the surface of activated carbons (for PRBs the
pH of the corresponding groundwater is assumed), and pK
a
-values of the
adsorbents are important to assess the efficiencies of PRBs. A comparison
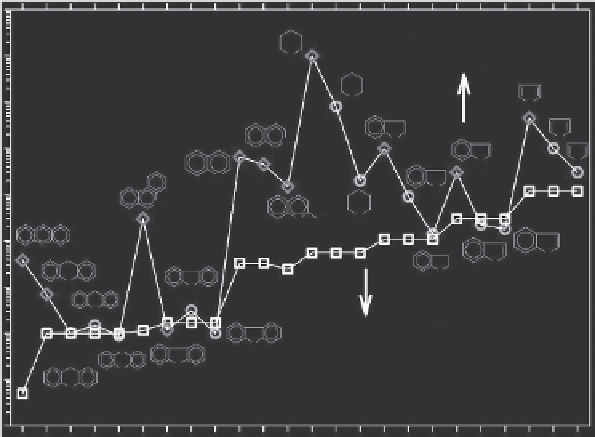
of the solubilities of NSO-heterocycles and their parent hydrocarbons and
PAHs is shown in Figure 11.1.
10
7
10
6
N
Heterocycles
10
5
O
N
O
N
H
10
4
N
N
H
S
N
10
3
O
N
S
N H
3
10
2
N
S
O
S
10
1
N
H
O
S
Aromatics and PAH
10
0
S
O
N
H
O
10
-1
O
10
-2
FIGURE 11.1
A comparison of water solubilities of representative NSO-heterocycles (neutral molecules)
and their corresponding parent aromatic compounds. (Experimental data from EPI-Suite U.S.
Environmental Protection Agency. 2012. Estimation Programs Interface Suite
™
for Microsoft
®
Windows, v 4.11.)


Search WWH ::

Custom Search