Environmental Engineering Reference
In-Depth Information
5.2 Results and Discussions
In contrast to numerous other technical ZVI and NZVI brands, carbonyl
micro-ZVI basically shows a low reactivity regarding the reductive deha-
logenation of perchloroethene (PCE) in groundwater. This can be readily
understood because it consists highly pure iron, and it is well known to be
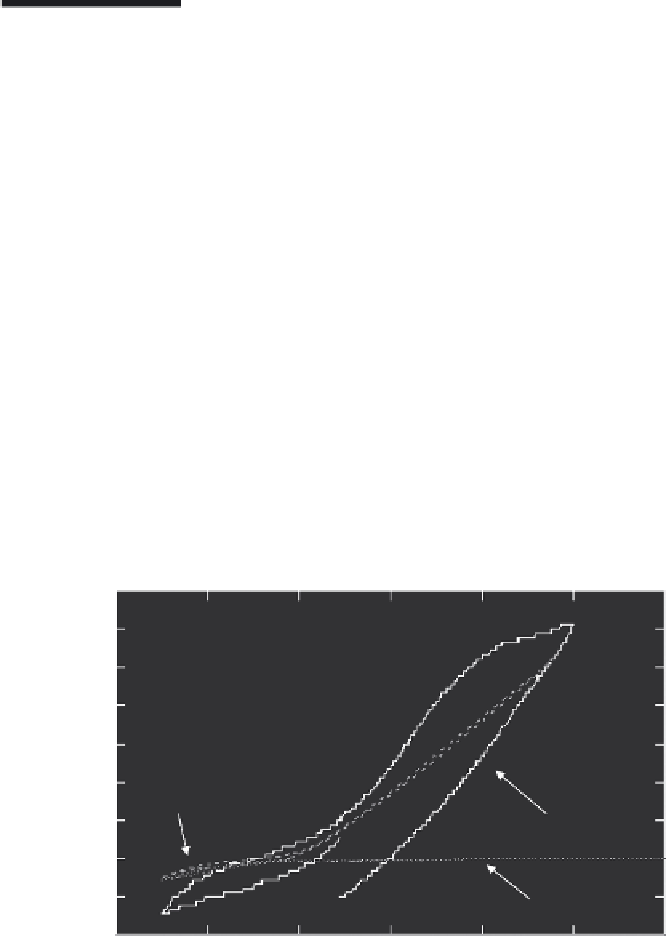
reluctant to corrosion in water at a higher degree. A cyclovoltagram (CV), as
shown Figure 5.1, shows that an iron electrode made of pure iron is much
nobler than technical grade iron such as iron sponge (Responge
®
) that has
been degrading the groundwater contamination of several 1000 μg/L PCE
in a pilot-scale ZVI PRB in Rheine, Northwest Germany, since 1998. It can be
seen that the slope of the hysteresis loop is much steeper for Responge; more-
over, the CV of pure Fe shows passivation in the potential range between −1.0
and roughly −0.7 V (related to a saturated calomel electrode [SCE]).
The CV measurements carried out with contaminated groundwater from
the Rheine site showed no evidence of direct PCE reduction signals (neverthe-
less, they can be found through measurements in aprotic solvents, e.g., acetoni-
trile). Therefore, reductive dechlorination of cVOCs in water by elemental iron
is, compared to anaerobic corrosion, a significantly less favorable process from
an electrochemical viewpoint.
14.000
12.000
10.000
8.000
6.000
4.000
Fe (pure)
2.000
Responge
0.000
-2.000
Pt
-4.000
-1400.0
-1000.0
-600.0
-200.0
E vs. SCE (mV)
200.0
600.0
1000.0
FIGURE 5.1
CV of a pure iron electrode in comparison to a platinum electrode and technical ZVI Responge
electrode in groundwater (collected at the Rheine PRB site in Germany, being contaminated
approximately by 10 mg/L PCE). SCE (saturated calomel electrode): +241 mV versus SHE (stan-
dard hydrogen electrode), mV = millivolts.
Search WWH ::

Custom Search