Biology Reference
In-Depth Information
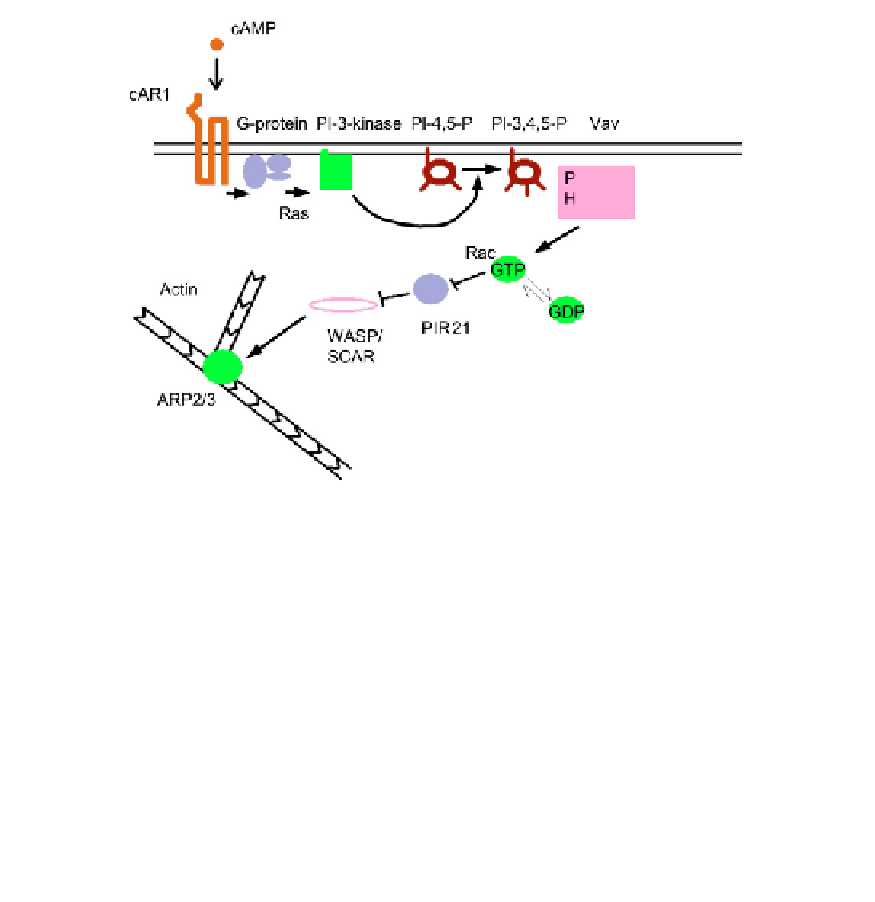
FIGURE 9.6
Link between the cAMP sensing pathway, via PI-3,4,5-P
3
, to the control of lamellipodial actin.
Other pathways, such as the TorC2 pathway, act in parallel with PI-3-kinase one and also encourage formation of
the leading edge.
19
The available evidence suggests that long-range inhibition of leading edge production is
mediated by cyclic GMP which, like cyclic AMP, has a high coefficient of diffusion and will
therefore travel quickly throughout the cytoplasm. The cytoplasmic concentration of cGMP
is controlled by the opposed activities of guanyl cyclase, which synthesizes it, and phospho-
diesterases that destroy it. Guanyl cyclase is activated by the cAMP sensing system, although
the details of this link are not yet known. The dynamics of activation are unusual, in that pre-
senting a cell with a constant concentration of external cAMP causes a transitory rise in internal
cGMP, which then falls away; only if the cell is presented with a steadily rising concentration
of external cAMP does the concentration of internal cGMP stabilize.
20
Effectively, the cGMP
system computes the differential of external cAMP with respect to time:
d
dt
½
cAmp
This connection with the time domain is probably very important to navigation, and will be
discussed further below.
One of the effects of cGMP is activation of cGMP-dependent myosin light chain kinases
that activate myosin and allow it to form filaments that associate with actin.
21
The formation
of these filaments, which are seen in the lateral and trailing edges of migrating cells (in other
words, everywhere except for the protrusive leading edge), is important for the restriction of
protrusive activity. As long as actin is organized as actin/myosin filaments, formation of
a branched protrusive network is inhibited; part of this activity is due to the ElmoA protein,
which associates with myosin and which inhibits new actin polymerization.
22
Once this
½
cGHP
a