Biology Reference
In-Depth Information
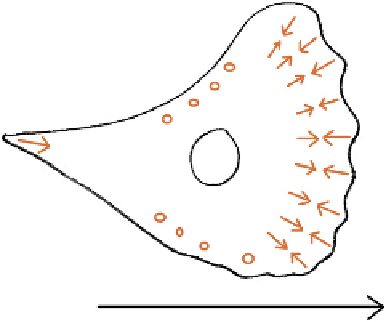
FIGURE 8.8
Tensions exerted on the substratum by a migrating cell. Arrows show the direction of tension,
while circles are placed on areas in which there seems to be no reproducible direction of tension. The cell is
advancing in the direction of the large arrow on the substrate.
a net rearward motion, the filaments in the cell body near the lamellipodium are stationary
with respect to the substrate and even move forwards in the heart of the cell body and its
trailing edge.
37
There is also little evidence of constant and directed traction in the cell
body except at the very tip of the tail, which pulls on the substrate as if it is reluctant to let
go as it follows the cell body forward.
36,38
Together, these observations suggest that tension
generated against the substrate adhesions just behind the lamellipodium pulls a rather
passive cell body along.
Mechanical attachment of cells' actin cytoskeletons to the substrate is usually via integrin-
containing junctions called 'focal adhesions'. As the lamellipodium advances, new focal
adhesions are formed. Their development requires Rac or cdc42 activity,
29,39,40
but it is not
clear whether this is a direct requirement or an indirect one in which Rac is needed for forma-
tion of new lamellipodium, and new lamellipodium is needed for formation of focal adhe-
sions.
41
The new focal adhesions formed at lamellipodia are smaller than those in the cell
body but they exert much stronger forces on the substrate than do the larger and mature
adhesions in the cell body,
42
providing further evidence that the cell is pulled from its leading
edge (or the region just behind it) and that the cell body itself produces little propulsive force.
Although the nascent focal adhesions include many of the same proteins found in all focal
adhesions (for example, talin, paxillin and low levels of vinculin and FAK)
42
, they are devoid
of some 'normal' components such as zyxin. They also form connections with microtubules
that would be expected, from the tensegrity model, to be in compression.
43
The organization of actin into tension-generating structures is under the control of another
small GTPase of the Rac family, Rho. Rho encourages formation of stress fibres by a variety of
means, which include regulation of filament length and regulation of tension itself. Activated
Rho inhibits cofilin (via LIMK), which would otherwise depolymerize actin; Rho therefore
encourages the accumulation of long actin fibres.
44,45
Rho also acts via ROCKs (Rho-kinases)
to phosphorylate, and therefore activate, myosin light chains by both stimulating myosin light
chain kinase and by inhibiting myosin light chain phosphatase.
46
e
48
Rho therefore increases
the tension in the stress fibre system and, because of the positive feedback involved in the