Biology Reference
In-Depth Information
would be difficult to explain if microfibrils were the source of alignment information. The
biochemical evidence comes mainly from experiments in which microtubule-depolymerizing
drugs are used to perturb the microtubule systems of plant cells, and their effect on cell elon-
gation and cellulose alignment is studied. In some plant cells, treatment with colchicines
causes the alignment of microfibrils to become random
d
as would be predicted from the
microtubules-align-microfibrils model.
11
ignment remains across
areas of cells but the global alignment across cells and tissues is lost.
12
If A. thaliana embryos
are grown in agar that contains the microtubule depolymerizing drug propyzamide, the elon-
gation of their cells becomes disregulated so that they grow in a spiral rather than straight
manner.
9
This supports the idea that the default orientation of microfibrils is spiral, and that
microtubules are normally used to steer the system away from its default. The microtubule
depolymerizing drug olyzalin has a similar effect onmicrofibril orientation in growing roots.
13
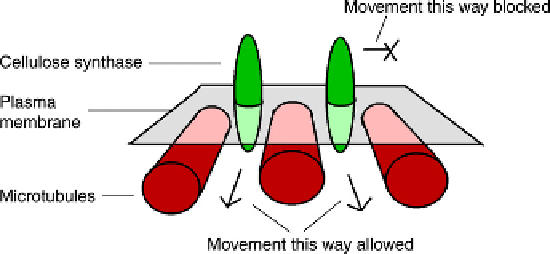
If the orientation of microtubules does determine the orientation of cellulose microfibrils,
the connection between the two may be explained by a simple model that uses the fact that
cellulose synthase molecules have to move as they do their work. Parallel arrays of microtu-
bules just under the membrane will impede the passage of a cellulose synthase complex
across them but will not impede its passage parallel to them (
Figure 6.3
). With all of the cellu-
lose synthase enzymes constrained to travel in the same direction, the cellulose microfibrils
will have to be laid down in this direction and the required anisotropy will be built into the
cell wall.
14
Little is known about precisely how microtubules are patterned according to the general
growth axis of the tissue. Microtubules seem to be nucleated in the cell cortex itself
15
and are
later cut free from their nucleation sites by the protein Katanin. They then show the '
In other systems, local a
l
' end
dynamic instability typical of microtubules (Chapter 5), and also show a slow shortening
from their '
þ
' ends. Their centre of mass therefore translocates, on average, towards the
' direction and they can therefore disperse throughout the cortex
16
(
Figure 6.4
). When
they meet, they can be cross-linked into bundles by a variety of microtubule binding proteins
such as MAP65 and MOR1.
15
Microtubules are also linked to the membrane itself, and the
most likely candidate for the linker is, surprisingly, phospholipase D.
17
Activating this
enzyme pharmacologically, by adding n-butanol to cells, causes cortical microtubules to be
released from the plasma membrane over the course of minutes.
18
'
þ
Inhibiting the enzyme
FIGURE 6.3
A mechanical model
for the guidance of cellulose synthase
complexes by cortical microtubules.
The movement of the synthase
complexes will determine the orienta-
tion of the cellulose polymers they leave
behind. This model depicts a passive
mechanical constraint: it is possible that
the synthase complexes are linked to
microtubules via kinesin-type motors
and are therefore guided along them.