Biology Reference
In-Depth Information
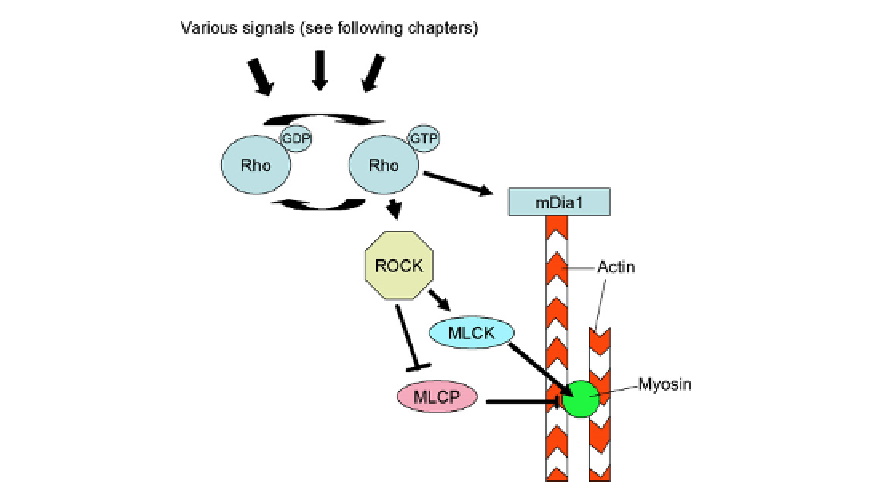
FIGURE 5.9
The small GTPase, Rho, controls microfilaments by regulating both their assembly and their ability
to generate tension. Rho is itself controlled by Guanidine nucleotide exchange factors, which move Rho into its
activated, GTP-bound state, and GTPase activating proteins that promote the reverse transition. These proteins are
themselves modulated by extracellular signals, as illustrated in later chapters. In this diagram, as in the rest of the
topic, arrows (
Y
) imply activation and blocks (
t
) imply inhibition.
developed by microfilaments. ROCK activates myosin light chain kinase,
40
which phosphor-
ylates myosin light chain and activates the tension producing activity of myosin. ROCK also
phosphorylates the myosin binding subunit of myosin phosphatase and consequently inac-
tivates it; this prevents myosin phosphatase from dephosphorylating the myosin light chain
and, by preventing this, it maintains myosin in an active state.
41
ASSEMBLY OF THE MICROTUBULE SYSTEM
Microtubules are polymers of tubulin molecules, which themselves consist of a hetero-
dimer of
a
-and
b
-subunits. Tubulin molecules are asymmetric, having ends conventionally
designated '
polarity. Tubulin monomers
have an affinity for guanosine phosphates and normally bind either two molecules of
GTP, one on each protein subunit, or one molecule of GTP and one of GDP. In the latter
case, the GTP is bound to the
a
-tubulin subunit, where it is buried and stable, and the
GDP is bound to the
þ
'and'
', and their polymers retain this
þ
/
-subunit has an inherent weak GTPase activity, so
that GTP that is bound to it is hydrolysed to GDP and P
i
.
42
The GDP so formed can be
exchanged with GTP in the cytoplasm surrounding the tubulin, and the cycle repeats (at
the cost of the energy required to synthesize GTP). The GTP bound to the
b
-subunit. The
b
-subunit is
buried and cannot be exchanged; it will therefore be ignored for the rest of this discussion
a