Biology Reference
In-Depth Information
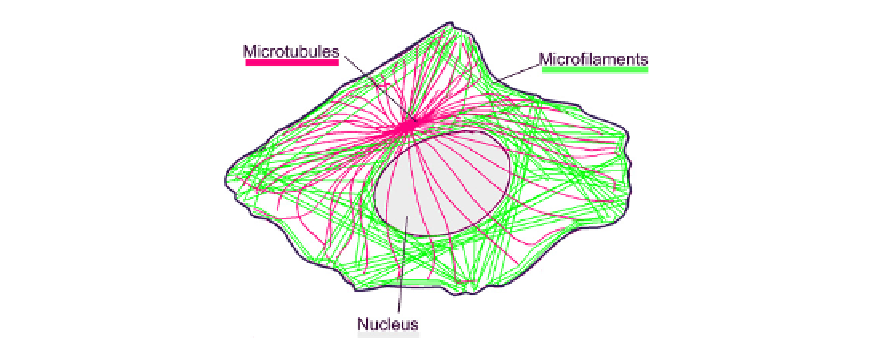
FIGURE 5.3
The principal tension and compression structures in a cell. Actin microfilaments (green) are
generally in tension and microtubules (red) are generally in compression.
matrix
5
(
Figure 5.3
). These divisions of duty are not completely clear-cut because actin bears
compressive forces at the leading edge of a cell (Chapter 8) and in protrusions such as micro-
villi (see below). The shapes of typical actin and tubulin cytoskeletons are compatible with
the roles proposed for them in tensegrity structures; microfilaments are usually straight as
would be expected for a filament under tension, whereas microtubules are usually curved
as would be expected for a strut under compression. Evidence for this balance of forces is
provided by experiments in which one component is removed. If, for example, spread cells
are removed from their matrix, they tend to lose their shapes and become spherical, suggest-
ing that having a substrate in compression and able to resist the tensile forces from the inside
of the cell was critical to their former shape. Similarly, if microtubules are depolymerized
with drugs so that they are no longer able to share the burden of compressive loads, the force
on the extracellular matrix is increased.
8
According to the tensegrity model, the shape of a cell is governed mainly by the arrange-
ment of and forces within microfilaments, microtubules, adhesions and the matrix (assuming
that the surface tension of the membrane is constant). Understanding cell shape therefore
mainly boils down to understanding the placement of elements of the cytoskeleton.
Cytoskeletal elements have to be located with an accuracy in the
50 nm range, partic-
ularly when the structures of adjacent cells connect via junctions. The spatial resolutions
offered by the patterning mechanisms of classical developmental biology are nowhere
near as fine as this; differentiation state varies cell-by-cell (spatial resolution in the 10
m
m
range) while gradients of morphogens such as retinoic acid can be read with an accuracy
no greater than a few
m
m, for reasons explored in Chapter 9. The embryo solves this
problem by using the 'classical' mechanisms of developmental biology, such as induction
of gene expression, only to invoke the building of molecular machines at the right time
and place. It then relies on the ability of these machines to build appropriate fine-scale
structures autonomously. Thus, almost every aspect of the fine-scale structure of an embryo
is the result of adaptive self-organization rather than specific 'programmed' spatial
planning.