Biology Reference
In-Depth Information
system, though it is known to require the kinase JNK.
4
In a broadly analogous experimental
system, the healing of a wound hole in the epidermis of D. melanogaster larvae, JNK is
required for proper location of myosin to the leading edge of the cell.
5
Rac and cdc42 can
lead to inhibition of the actin-severing protein, cofilin, and this may also assist the assembly
of actin-myosin cables.
6
Tension in the actin-myosin cables is one direct mechanism for drawing the sides of the
hole together (
Figure 17.2
b). The importance of this myosin-mediated contraction is under-
lined by the observation that mutant embryos that have no myosin II heavy chain fail to
complete dorsal closure,
7
although this does not in itself prove that contraction of the leading
edge is the essential event for which myosin II is needed. Laser ablation of a small number
(2
e
3) cells at the leading edge of the hole causes the cells adjacent to their flanks to spring
apart sideways, providing further evidence that the alternating band of actin and cell junc-
tions around the hole really is under tension.
1
Laser ablation of leading edge cells does
not, however, block dorsal closure even if multiple sites are ablated (
Figure 17.3
b). Even
though the advance of a site of ablation is slowed down temporarily, it catches up before
long. Other mechanisms must therefore be capable of drawing the sides of the hole together.
The leading edge of the advancing epidermis is atypical, by the usual standards of epithe-
lial tissue, in that it represents a 'free' lateral edge (the lateral edges of most epithelial cells are
in contact with neighbouring epithelial cells). This free edge is largely free of the normal
apico-basal polarization of conventional epithelial cells
8
and has a propensity to behave
like the leading edge of a migratory cell and to produce lamellipodia and filopodia. In fibro-
blasts, Rho antagonizes the production of these motile structures (see Chapter 8), and the
same thing seems to happen in epithelial cells taking part in dorsal closure: wild-type cells
show restrained motility but Rho mutants show much more lamellipodial and filopidal
activity.
3
This may provide the embryo with an automatic backup system for dorsal closure:
if the Rho-driven actin belt system is working normally, then cells will move by their circum-
ferential tension but, if Rho-activated actin belt formation fails, then the cells produce
machinery to migrate forwards by direct cell locomotion. Mosaic experiments, in which
dominant negative Rho is expressed by groups of cells surrounded by wild-type neighbours,
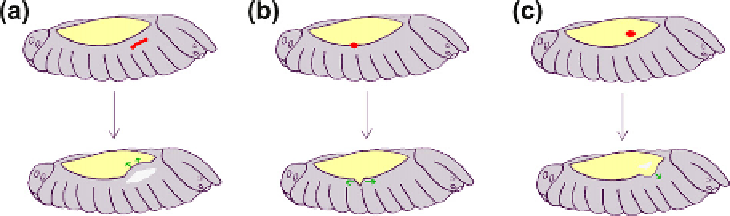
FIGURE 17.3
Effects of laser ablation on dorsal closure in D. melanogaster. The site of ablation is shown as a red
spot and resulting movements by green arrows. (a) Ablation of a region of epidermis away from the edge increases,
rather than decreases, progress towards the midline suggesting that progress cannot be driven by pushing from
behind. (b) Ablation of cells at the leading edge causes their neighbours to spring apart, as if under tension. (c)
Ablation of an area of amnioserosa delays progress, as if the amnioserosa is pulling on the epidermis.