Environmental Engineering Reference
In-Depth Information
4.4.1.4 Future of LA battery
Due to the low cost and maturity of the LA battery it will probably always be useful
for specifi c applications. The international
Advanced Lead-Acid Battery Consor-
tium
is also developing a technique to signifi cantly improve storage capacity and
also recharge the battery in only a few minutes, instead of the current hours [2].
However, the requirements of new large-scale storage devices would signifi cantly
limit the life of a LA battery. Consequently, a lot of research has been directed
towards other areas. Therefore, it is unlikely that LA batteries will be competing
for future large-scale multi-MW applications.
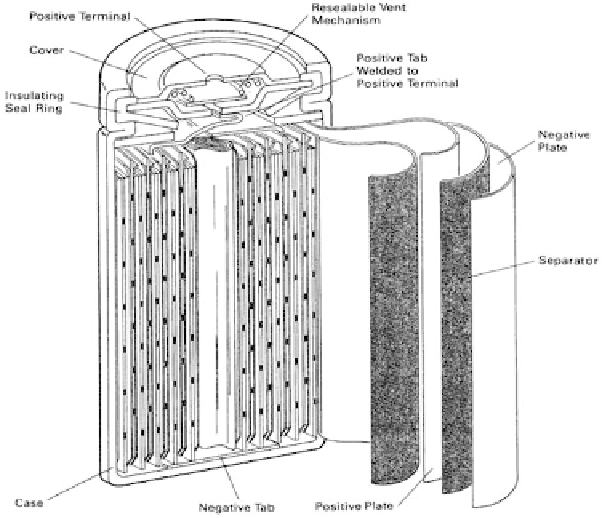
4.4.2 NiCd battery
A NiCd battery is made up of a positive with nickel oxyhydroxide as the active
material and a negative electrode composed of metallic cadmium. These are sepa-
rated by a nylon divider (see Fig. 7). The electrolyte, which undergoes no signifi -
cant changes during operation, is aqueous potassium hydroxide. During discharge,
the nickel oxyhydroxide combines with water and produces nickel hydroxide and
a hydroxide ion. Cadmium hydroxide is produced at the negative electrode. To
charge the battery the process can be reversed. However, during charging, oxygen
can be produced at the positive electrode and hydrogen can be produced at the
negative electrode. As a result some venting and water addition is required, but
much less than required for a LA battery.
Figure 7: Nickel-cadmium battery [ 16 ].
Search WWH ::

Custom Search