Biomedical Engineering Reference
In-Depth Information
where
C
is the concentration and the subscripts A, F, P, and W represent ascorbic acid, lauric
acid, lauroyl ascorbate, and water, respectively. The subscript e indicates the equilibrium. The
values of
C
Pe
and
C
We
were experimentally determined. As shown in Figures 3(a) and 4(a),
the conversion increased with time, reached a plateau and then decreased gradually due to
degradation of the product. Since the degradation rate was much slower than the formation
rate, the maximum conversion was regarded as the equilibrium conversion. The
C
Ae
values
were estimated from the solubility of ascorbic acid in used organic solvent. The
C
Fe
values
were evaluated by subtracting
C
Pe
from the initial concentration of lauric acid
C
F0
. The plots
of
C
Pe
C
We
versus
C
Ae
C
Fe
gave a nearly straight line passing through the origin with the
correlation coefficient of 0.95, and the equilibrium constant
K
C
was evaluated to be about 1.6.
2. Continuous Production of Acyl Ascorbate Using
a CSTR or PFR
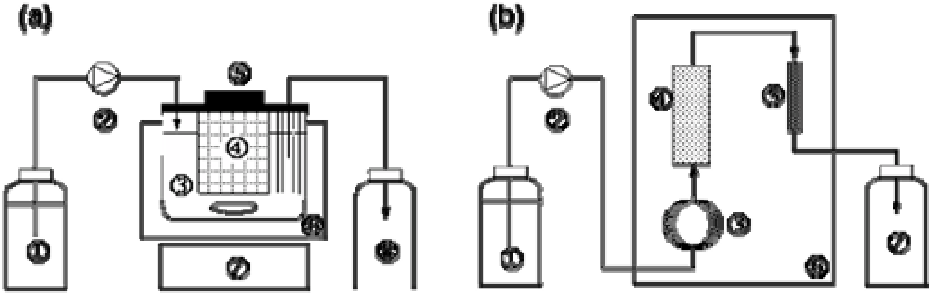
A schematic diagram of a continuous stirred tank reactor (CSTR) system is shown in
Figure 5(a) [29]. An immobilized lipase (3.0 g by dry weight), Chirazyme
®
L-2 C2, was
packed into a stainless steel basket. The volume of the solvent in the reactor was 350 mL. At
the beginning of the operation, 40 g of ascorbic acid was added to the reactor after removing
the upper lid. A fatty acid (decanoic, lauric or myristic acid) was dissolved with acetone at the
concentration of 200 mmol/L, and the mixture was fed to the reactor (85 mmφ × 90 mm) at a
specified flow rate. The reactor was immersed in a water-bath at 50
o
C and the reaction
mixture was mixed by a magnetic stirrer. The flow rate was varied in the range of 1.5 - 9.9
mL/min for each fatty acid.
A scheme of a plug flow reactor system (PFR) is shown in Figure
5(b) [31]. Ascorbic acid powders (
ca.
40 g) were packed into a cylindrical glass column (10
mmφ × 150 mm) and Chirazyme
®
L-2 C2 particles (
ca.
1.5 g by dry weight) were packed into
a stainless column (4.6 mmφ × 150 mm). The columns were connected in series with a
stainless steel tube. A fatty acid (decanoic, lauric, myristic, oleic, linoleic or arachidonic acid)
was dissolved in acetone at a concentration of 25 - 250 mmol/L. The fatty acid solution was
Figure 5. (a) Scheme of a CSTR for the continuous synthesis of saturated acyl ascorbates; 1: feed
reservoir, 2: pump, 3: reactor, 4: basket packed with immobilized lipase, 5: lid, 6: water-bath, 7:
magnetic stirrer, 8: effluent reservoir; (b) Scheme of a PFR; 1: feed reservoir, 2: pump, 3: pre-heating
coil, 4: column packed with ascorbic acid, 5: column packed with immobilized lipase, 6: thermo-
regulated chamber, 7: effluent reservoir.