Biomedical Engineering Reference
In-Depth Information
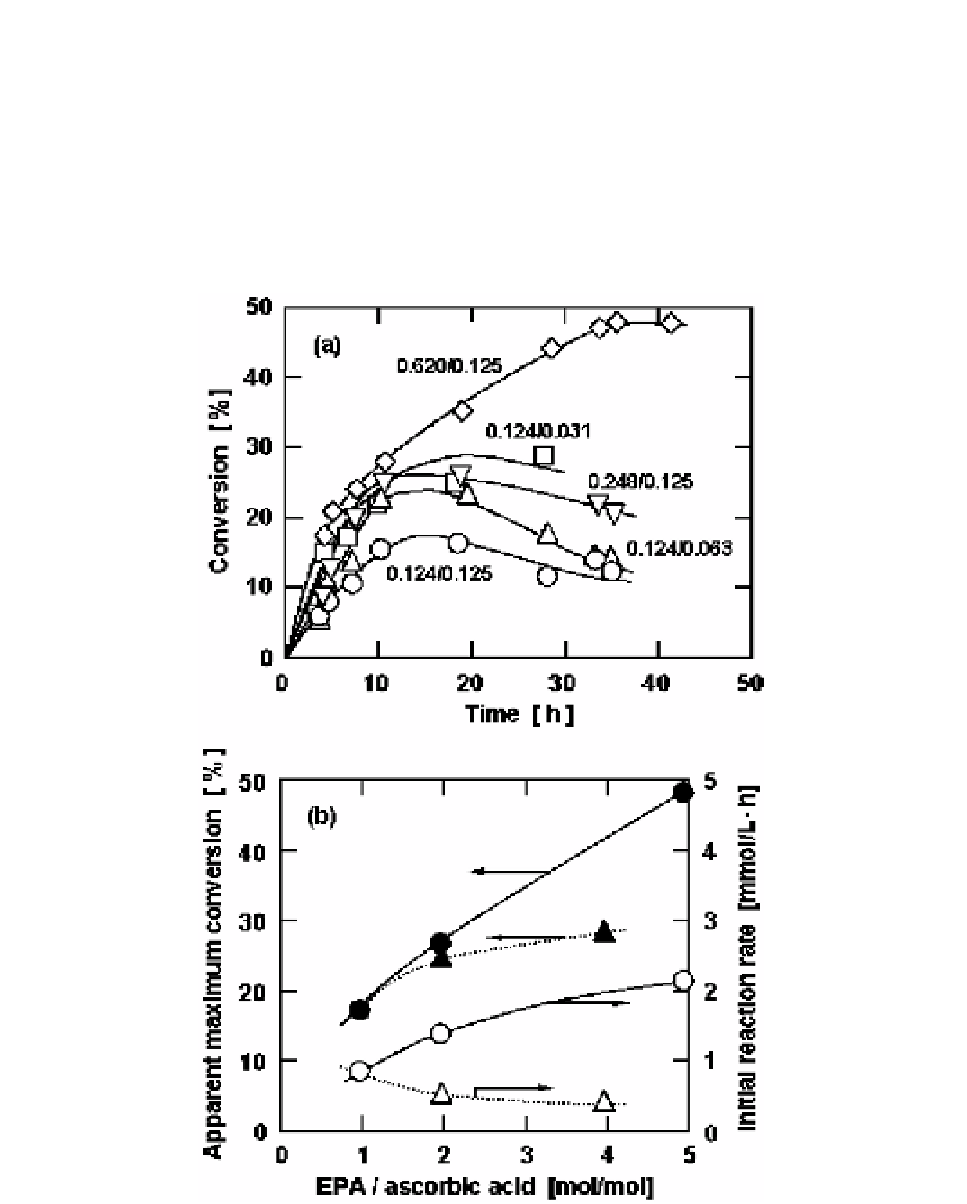
ascorbic acid was changed (open triangle), the reaction rate became slower at the higher ratios
due to the decrease in the initial ascorbic acid concentration. The relationships between the
maximum conversion and the molar ratio for the condensations of ascorbic acid and saturated
fatty acids with various chain lengths from 6 to 12 were examined [24]. There was no
significant difference in the relationship among the fatty acids tested. The maximum
conversion was higher at the higher molar ratio. Since high conversion and a lower amount of
unreacted fatty acid were favorable for the purification process, the molar ratio of 5 would be
the most appropriate for production of acyl ascorbate in the present reaction system.
Figure 3. Lipase-catalyzed condensation of eicosapentaenoic acid (EPA) and ascorbic acid at their
various molar ratios in acetone at 55
o
C. (a) Time courses for conversion of 6-
O
-eicosapentaenoyl
ascorbate: the amounts of EPA/ascorbic acid (mmol/mmol) added in 2.5 ml acetone are shown in the
figure. (b) Dependence of apparent maximum conversion (closed symbols) and initial reaction rate
(open symbols) on the EPA/ascorbic acid molar ratio. Circles and triangles represent the results
obtained at the fixed amounts of ascorbic acid (0.125 mmol) and EPA (0.124 mmol), respectively. The
curves were empirically drawn.