Biomedical Engineering Reference
In-Depth Information
1
1
x
Α
,in
bil
0.7
0.3
0
0.8
K
0
A/Q
Α
c
alb
=1
0.8
0.6
0.6
0.4
0.4
x
Α
,in
bil
K
0
A/Q
Α
c
alb
=0.1
0.2
0.2
0.7
0.3
0
0
0
0
1
2
3
4
0
0.5
1
1.5
2
K
0
A/Q
alb
c
alb
Q
Β
c
alb
/Q
Α
c
alb
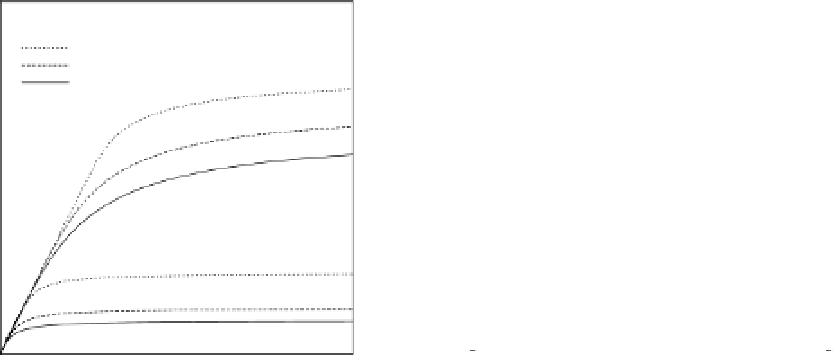
Figure 3. Clearance of strongly albumin-bound toxins obtained by albumin dialysis with
fresh dialysate (
X
Β,IN
= 0
). On the left: predicted values with different operating condi-
tions. On the right: limiting values (
1/Z→∞
) for different inlet toxin concentrations.
• if the solution to be detoxified has a very low bilirubin-to-albumin molar ratio, i.e. in
the limit of
X
Α,IN
→0
, equations (22) and (23) can be analytically integrated to give:
CL
Q
Α
1−exp [Κ (1−Z)]
Z−exp [Κ (1−Z)]
=
(27)
This limiting expression for the module clearance underestimates the exact solution
when
X
Α,IN
> 0
and can therefore be considered as a conservative approximation. It
is worth noting that, in these conditions, the driving force for toxin transfer is
X
Α
−X
Β
,
coherently with the empirical findings reported by Dammeir et al. (2007).
The order of magnitude of
K
0
A
for the membrane modules used in albumin dialysis LSDs
can be estimated by the data reported by Stange and Mitzner (1996) and referring to the
time course of bilirubin concentration in a two-compartment, closed-loop dialysis system.
The model presented in this section, was coupled with mass balances for the two compart-
ments and fit against these experimental data. The optimal value found for
K
0
A
was 2.61
Μ
mol/min.
4.
Adsorption Process
In recirculating albumin dialysis devices such as MARS, adsorption is one of the fun-
damental steps in dialysate regeneration. Fixed bed units are normally used for this task,
and the design of such units requires information both on toxin adsorption equilibrium and
kinetics.
The most common adsorptive media used in LSDs are activated carbon, non-ionic poly-
meric resins and anionic resins.
Bilirubin adsorption equilibrium on these media from