Biomedical Engineering Reference
In-Depth Information
into traditional polyacrylamide gels may produce homogeneous molecular imprinted
polymers for proteins with a fine affinity, selectivity and rebinding capacity. Using randomly
distributed carboxylic groups on the backbone of the polymer chain as recognition sites, Zhao
et al. [36] prepared molecular imprinted polymer with cloned bacterial protein template to
enrich authentic target in cell extract. It seems that, in this case, the electrostatic force and
cavity shape were the dominate factors for the selective recognition.
It has been suggested that binding in aqueous solution can be enhanced by exploiting
cooperative interactions and selecting proper hydrophobic microenvironments to create better
receptors. In one of most interesting example of molecular imprinting by the cooperative
interactions of hydrophobic and electrostatic interaction, acrylated β-cyclodextrin was
copolymerized with an electrostatic functional monomer 2-acryoylamido-2,2′ -
dimethylpropane sulfonic acid. β-cyclodextrin has a hydrophobic cavity which can bind
aromatic groups on the chain of biomacromolecules. So the hydrophobic effect is the
dominant factor in imprinting water rich system, whereas electrostatic interaction dominate
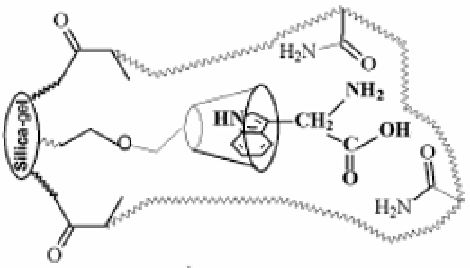
recognition in aqueous poor mixtures [37, 38]. Similarly, Qin et al. [39] pointed out that
bonded β-cyclodextrin and acrylamide cooperated together could improve the selective
recognition ability of the molecular imprinted polymers for the template tryptophan. Figure 1
showed the formation of template-receptor complexation. The high performance liquid
chromatography column packed with this kind of molecular imprinted polymer could even
separate the template from its enantiomer in aqueous mobile phase. Using toluene
diisocyanate as the cross-linking agent, Egawa et al. [40] and Asanuma et al. [41] prepared
molecular imprinting cyclodextrin polymers in the present of cholesterol template. Although
the imprinting occurred in a dimethylsulfoxide emulsion, the recognition could be achieved in
the solvents containing water since binding of the guest molecules was based on inclusion
complex formation with cyclodextrins. Thus, these polymeric receptors are not only
applicable to selective recognition of biologically important molecules, but also removal of
toxic molecules from aqueous media.
Figure 1. Schematic representation of tryptophan template imprinting.
In contrast to weak interactions like hydrogen bonding and hydrophobic interactions in
water, metal complexation [22, 42] and ionic interaction [43] would provide much stronger
interactions for molecular recognition. Janotta et al. [44] demonstrated that ionic interaction
can play an important role during the selective recognition of 4-nitrophenol by molecular
imprinted polymers in aqueous solution. Interestingly, polymers imprinted with 2-nitrophenol
and 3-nitrophenol show less pronounced imprinting effects comparing the 4-nitrophenol
imprinted polymer. The decrease of the imprinting effect may be result from the fact that the