Biology Reference
In-Depth Information
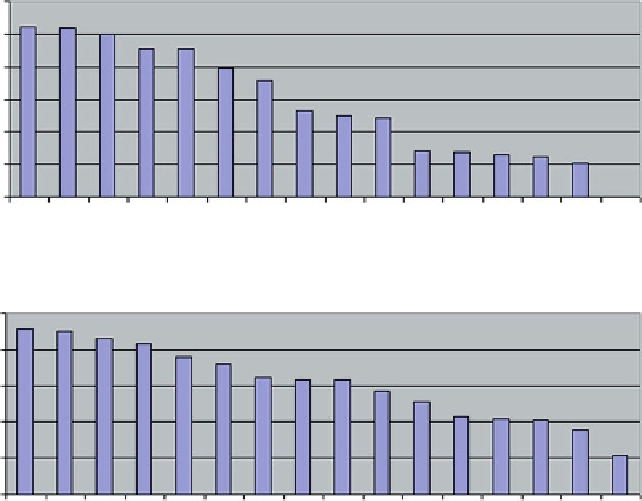
ROSETTA-Dock F-measure LOWEST
0.12
0.1
0.08
0.06
0.04
0.02
0
ROSETTA-Dock MCC LOWEST
0.2
0.1
0
-0.1
-0.2
-0.3
Fig. 6.13
Worst solutions produced by RosettaDock, ranked according to F-measure (
top
) and
MCC (
bottom
) criteria. Only chains A are shown
The “fuzzy oil drop” model identifies complexation sites as specific deforma-
tions in the protein's hydrophobic core associated with the presence of residues
whose actual hydrophobicity values diverge from theoretical predictions. When the
core is perturbed by more than one external molecule (for instance by a protein and
a ligand), it becomes difficult to distinguish one distortion from the other. Thus,
accurate prediction of ligand binding and protein complexation sites depends on
measuring the relative significance of each factor.
For the sample protein designated 1G8M (transferase, hydrolase - crystal struc-
ture of avian atic, a bifunctional transformylase and cyclohydrolase enzyme in purine
biosynthesis - EC 2.1.2.3, EC 3.5.4.10) (Greasley et al
2001
) the “fuzzy oil drop”
model was able to correctly identify the complexation site (by locating residues
which represent local maxima of the
Δ
H
profile). However, this protein is also capable
of binding a ligand (specifically, C
10
H
14
N
5
O
8
P - Guanosine-5¢ -monophosphate).
Identifying this ligand's binding pocket would likely prove dif fi cult as the deformation
Search WWH ::

Custom Search