Biology Reference
In-Depth Information
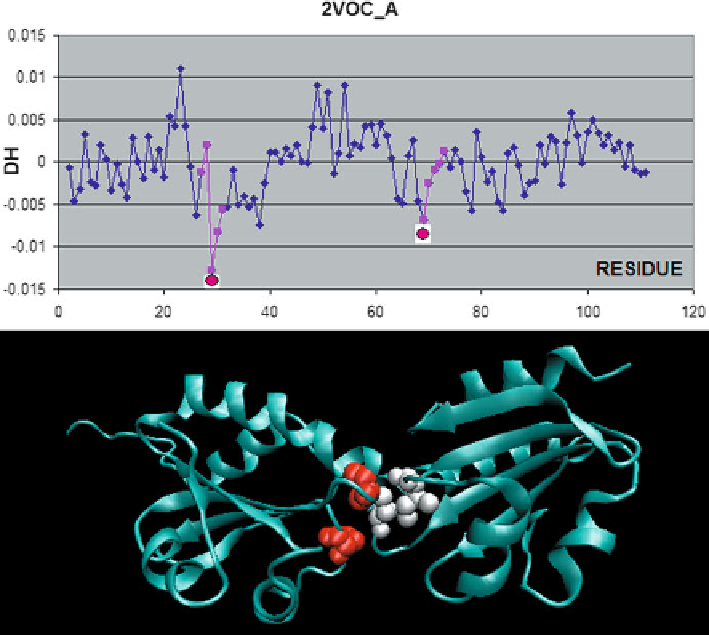
Fig. 6.1
Thioredoxin homodimer complex (2VOC) with a mutated active center, composed of
mixed disulfide dimers which resemble the enzyme-substrate reaction intermediate.
To p
:
Δ
H
profile indicating residues engaged in complexation (according to PDBSum).
Bottom
: 3D ribbon
model with CPK representations of local
Δ
H
profile minima (highlighted in the
top
diagram)
Δ
H
expresses irregularities in the distribution of hydrophobicity,
which can manifest themselves as either localized deficiencies or excess of hydro-
phobicity. Deficiencies are typically associated with the presence of cavities in the
globular protein body, while excess hydrophobicity, when present on the surface,
indicates potential complexation sites. Such sites are naturally attracted to one
another - this interaction shields them from the entropically disadvantageous con-
tact with water, ensuring the formation of a stable complex.
Successful prediction of complexation sites via this mechanism is possible e.g.
for the thioredoxin A homodimer (2VOC) with a mutated active center composed
of mixed disulfide dimers, resembling the enzyme-substrate reaction intermediate
(Kouwen et al.
2008
). The biological role of this protein is to assist in electron trans-
port. Each monomer contains strongly hydrophobic Cys and Val residues, repre-
senting local minima of the
The value of
Δ
H
profile. Both residues are exposed on the protein
surface and therefore preferentially attract hydrophobic residues belonging to the
complementary monomer (Fig.
6.1
). The resulting dimer is a good example of the
applicability of our model.
Search WWH ::

Custom Search