Environmental Engineering Reference
In-Depth Information
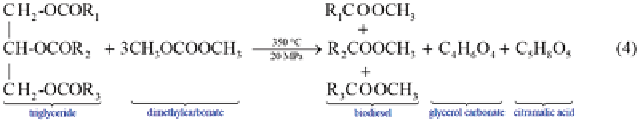
In Eq. (5.3), the esters formed from the reaction represent the biodiesel product
and the glycerol represents the by-product, which is not always desirable. In the
Saka series of methods to synthesize biodiesel, other reaction solvents offer inter-
esting possibilities. For example, when supercritical dimethyl carbonate is used as
the solvent, the by-product is glycerol carbonate and citramalic acid, which can be
considered to be value-added products:
Thus, there are many favorable combinations of supercritical organic solvents that
can lead to more desirable chemical products.
Many substances in their supercritical state can act not only as a reaction solvent,
but also become part of the product and by-product. Further, in their supercritical
state, organic solvents have much more favorable heat and mass transfer charac-
teristics compared with those of a liquid solvent and frequently catalysts are not
required.
5.3.10
Biomass Conversion
The Sun is the source of all of our sustainable energy with Earth receiving roughly
100,000 TW (75,320 Gtoe/year) of energy. The Earth's ecosystems only absorb a
fraction of this energy with the greatest recoverable amounts being stored in the
atmosphere as wind, in terrestrial ecosystems as plants and forests, and in aquatic
ecosystems as phytoplankton. Considering our projected energy consumption of
22.2 TW in 2040, harvesting only a small fraction of the available energy would
allow the world to eliminate energy poverty and improve human welfare. Develop-
ing efficient ways of converting biomass in its various forms are seen as important
topics for future sustainable development.
Biomass occurs in many different forms and is diverse, seasonal and unevenly
distributed in the world. Biomass has been used extensively throughout history for
a multitude of purposes. Most lignocellulosic biomass contains cellulose (ca. 50 %),
hemicellulose (ca. 25 %), lignin (ca. 20 %), inorganics (ash, ca. 2 %) and wax (ca.
< 1 %). Several strategies exist for converting biomass to chemical products: (i)
thermal, (ii) biochemical and (iii) chemical.
Thermal methods use heat to convert biomass to chemical products.
Torrefac-
tion
applies mild heat (ca. 200 °C) to biomass in the absence of oxygen to vaporize
light oils and moisture and produces densified material that is suitable for pelleting.