Environmental Engineering Reference
In-Depth Information
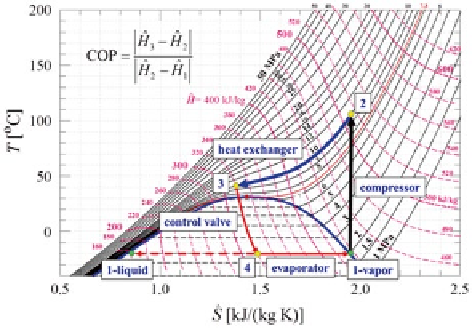
Fig. 5.5
Temperature-
Entropy (
T
-
S
) diagram for
CO
2
showing a typical set of
conditions for a heat pump
system. The COP of the heat
pump is given by the ratio
of enthalpy changes of the
fluid, namely, the enthalpy
change of the fluid in the heat
exchanger (
2 to 3
) divided
by the enthalpy change of
the fluid in the compressor
(
1 to 2
)
exchange between CO
2
in its supercritical state and water at ambient conditions
takes place from
Point 2
to
Point 3
in a heat exchanger. The heat exchanger allows
the heat of a hot fluid to be transferred to that of a cold fluid without direct contact
between the two fluids. For the case of making hot water, CO
2
transfers its heat
(105-40 °C) according to its decrease in enthalpy (510-320 kJ/kg) to heat water
(20-90 °C).
In Fig.
5.5
, the CO
2
remains in a single phase (supercritical) condition during
the heat transfer process from
Point 2
to
Point 3
. After the CO
2
transfers its heat, it
is expanded through a valve from
Point 3
to
Point 4
which causes both liquid and
vapor to form. The pressure chosen for
Point 4
controls the final temperature ac-
cording to the phase behavior of CO
2
. At
Point 4
, the temperature of the CO
2
is at
− 20 °C, which is considerably colder than the environmental temperature of + 20 °C
so that the environment can supply heat to the evaporator. Heat transfer from the
environmental allows the CO
2
to be recycled so that the process can be continu-
ous. Since one of the paths shown (
Point 2
to
Point 3
) uses the working fluid
(CO
2
) in the supercritical region, the energy system shown in the figure is called a
transcritical cycle
.
5.3.5
Cryogenic Exergy Recovery Energy Systems
Natural gas, which is primarily methane (CH
4
) is shipped worldwide as a liquid at
− 160 °C and atmospheric pressure. This liquefied natural gas is referred to as LNG
and in this form, its volume is reduced by about 1/630 compared with its gas form.
After the LNG reaches its destination at a terminal, it is typically heated with sea
water to convert it to vapor so that it can be transported through pipelines. The avail-
ability of a large cold temperature source is referred to as
cold energy
or
cryogenic
exergy
since its temperature differs substantially from that of the environment.
Natural gas requires about 850 kWh of electrical energy per ton to produce the
liquid that is necessary for its transport (Angelino and Invernizzi
2009
). The use
of heat pump cycles in the conversion of LNG to gas at receiving terminals has