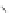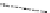Environmental Engineering Reference
In-Depth Information
Cathode
Electrolyte
Anode
Interconnect
(Segmented-in-series)
Planar
Porous substrate
Interconnect
Tubular
Interconnect
Anode
Flat Tubular
Electrolyte
Cathode
Anode
Cathode
Electrolyte
Cathode
Electrolyte
Interconnect
Anode
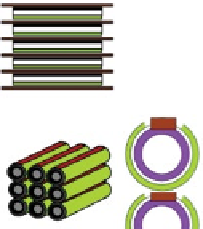
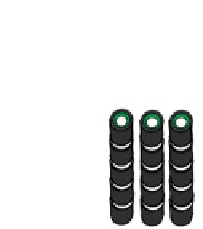
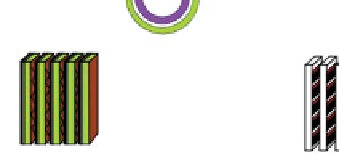
Fig. 4.10
Various types of SOFC stack
fuels are used
2
. So far, SOFC systems with energy conversion efficiencies rang-
ing from 40 to 60 % LHV have been developed. Even higher efficiency may be
possible when the exhaust heat is utilized for other types of generators, like a
fuel cell-gas turbine combined cycle, a triple combined cycle with an additional
steam turbine, or any other co-generation systems.
• Flexible fuel selection. SOFC can use a variety of fuels including H
2
and CO (a
reformer may not completely reform the hydrocarbon fuels, thus CO can be ac-
commodated, provided that carbon deposition is avoided).
• Difficulty in start/shutdown. Since SOFC is made of layered ceramics, fast heat
up and cooling down may cause mechanical damage, which would be a fatal
failure.
• Cost. The use of rather large amounts of nickel and rare earth metals contributes
to a high material cost for SOFC even though it does not use precious metals.
Further developments are under study to achieve lower costs and longer durability.
2
This may be confusing for a reader who has learned that the theoretical efficiency of a fuel cell,
Δ
r
G
/Δ
r
H
, decreases with increasing temperature. There are two reasons for the high efficiency of
SOFC. One is the small activation polarization in the electrode processes at high temperatures.
Another reason is related to the thermodynamics of hydrocarbon fuels. When methane is used, for
example, it is first reformed to hydrogen using water as CH
4
+ H
2
O = CO + 3H
2
. Since this reaction
is endothermic, heat must be obtained by using a part of fuel in the PEFC, but with a SOFC, high
temperature waste heat can be used for the reforming reaction, increasing overall efficiency.