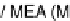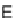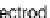Environmental Engineering Reference
In-Depth Information
Fig. 4.7
Schematic illustration of a single cell of PEFC
membrane. The negative electrode on which a hydrogen molecule is oxidized (two
electrons are extracted) to form protons is called the 'fuel electrode' or 'anode'. The
opposite electrode is called the 'air electrode' or 'cathode' which reduces protons
to a hydrogen molecule. The tri-layer of anode-electrolyte-cathode is referred to as
a 'single cell' or 'MEA' (membrane-electrode assembly) especially for PEFC. The
single cells are laminated via a current collecting plate (called a 'separator') made
from materials such as carbon, to build a 'stack' to generate a practical high voltage.
Cooling water is often circulated inside the separators to remove heat generated by
thermodynamic and kinetic losses. (Fig.
4.7
)
The energy conversion efficiency in a PEFC is limited by the activation resis-
tance at the electrodes, especially at the cathode. Although the theoretical equilib-
rium potential of a single cell is above 1.2 V, the output voltage is generally below
0.8 V when a practical current is extracted though, with a PEFC, further voltage
loss is small when a large current per area is extracted. The high current density,
together with the simple structure of the cell stack, thus enables PEFC to achieve
a high power density per unit of volume, which makes it suitable for fuel cell ve-
hicles. It is a common feature of PEFC to operate at ambient temperatures, and this
low operating temperature relative to other major types of fuel cells enabling quick
start-up and shutdown, is also advantageous for vehicles and other mobile applica-
tions (Fig.
4.8
).
Technical problems which relate to PEFCs can be summarized as follows:
• Poisoning of catalyst. When hydrogen fuel is contaminated with CO at 10 ppm
or above, this strongly adsorbs on the Pt catalyst in the electrode, and reduces its