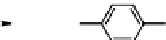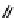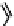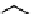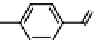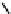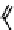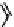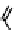Environmental Engineering Reference
In-Depth Information
*O\FRO\VLVE\WKH7HLMLQ3URFHVV
2
2
2
2
2+
+2
2+
+2
2
2
2
2
3(7
(WK\OHQHJO\FRO
%+(7
2
2
2
2
&+
2+
+2
2+
+
&
2 &
+
2
2
2
%+(7
0HWKDQRO
'07
2+
+2
(WK\OHQHJO\FRO
2
2
2
2
+
2
&
+
2
+
+
&
2
2 &+
+
2
2
+
a
'07
:DWHU
7HUHSKWKDOLFDFLG
0HWKDQRO
+\GURO\VLV
2
2
2
2
2+
+
2
+2
2
2
+2
2+
b
3(7
:DWHU
(WK\OHQHJO\FRO
7HUHSKWKDOLFDFLG
Fig. 11.7
Chemical reactions occurring during glycolysis and hydrolysis of PET
(hydrochloric acid or sulfuric acid). If sulfuric acid is used, high purity Na
2
SO
4
can
be obtained and sold, reducing the cost of the process. PET is readily dissolved in
concentrated sulfuric acid. However, recovery of terephthalic acid and purification
of sulfuric acid make this process expensive.
11.5.3
PET Composites
Composites are materials consisting of at least two different physically separated
materials with different material properties. Examples of plastic containing com-
posites are fiber reinforced plastics as they are used as light materials in plane and
ship building, and PET/PVC composites, in which PET fibers are embedded in PVC
sheets, giving strong weatherproofed tarpaulins.
Magnetic PET prepaid cards contain magnetite (Fe
3
O
4
) responsible for the
magnetic properties of the card and rutile (TiO
2
) as a white pigment. Both materi-
als together improve the mechanical strength. They are incorporated to an amount
of about 20 % (by wt.) in the PET matrix. Incineration of such cards leaves a
residue that is similar in composition to ilmenite, which is used as a basic ore in
the production of titanium; these minerals can be used in existing metal producing
facilities.
PET is of course lost during incineration and future research should focus on the
recovery of organic materials as well as the inorganic components. Recycling tech-
niques for PET as they were described above are not suitable for such composite